I know a guy who sells 100mg for $200, but only to people he likes LoL!
$215 is for a “kit” of ten vials, so that’s actually 300mg.
That is a good price ![]() the way it was written was not super clear on that.
the way it was written was not super clear on that.
I appreciate you chiming in to make sure I wasn’t drastically overpaying !
Just got this in an email from Merek Health (the blood test guys):
Is your Chinese Peptides Dealer Poisoning You?
One of the most significant forensic audits of the illicit peptide market was conducted by Janvier et al., titled “Impurity profiling of the most frequently encountered falsified peptide drugs on the Belgian market”.12 The researchers purchased samples from various grey market internet vendors and subjected them to rigorous Liquid Chromatography-Mass Spectrometry (LC-MS/MS) analysis.
Key Findings:
- Purity Failure Rates: Only 38.0% of the purchased peptides met the minimum purity requirements for pharmaceutical use.12 The majority were contaminated.
- Sequence Deletions: Many peptides had “deletion sequences”—peptides missing one or more amino acids from the chain.12 For example, a growth hormone secretagogue peptide sequence requiring 29 amino acids might be sold with only 28- or 27-amino acids. These truncated peptides may be biologically inactive, or worse, act as competitive antagonists (blockers) at the receptor site, negating any therapeutic benefit.
- Chemical Modifications: The study found extensive oxidation of amino acids like Tryptophan and Methionine.12 Oxidized peptides can have altered immunogenicity, potentially triggering the body to develop antibodies against the peptide (and potentially against the user’s own endogenous hormones).
The recent explosion in demand for GLP-1 agonists has exacerbated these quality control issues. A 2024 study (PubMed ID 39509151) specifically investigated the quality of semaglutide.2
The “Purity vs. Content” Paradox:
The study revealed a terrifying discrepancy between the advertised purity and the true purity. The measured purity of the semaglutide in the vials ranged between 7.7% and 14.37%.2 This means that over 85% of the solid mass in the vial was not semaglutide. The researchers found many samples with unknown synthesis contaminants.
When a consumer injects a grey market peptide, they are bypassing the body’s primary defense systems: the gut and the liver. This route of administration exponentially increases the toxicity of contaminants, particularly heavy metals.
The human gastrointestinal (GI) tract is a highly selective barrier. It limits the absorption of many toxins. For instance, the oral absorption of the heavy metal cadmium is estimated to be only about 5% of the ingested dose.14 Furthermore, blood from the gut passes through the portal vein to the liver, which acts as a filter (First-Pass Metabolism).
Injections bypass both protection mechanisms.
- 100% Bioavailability: Metals injected subcutaneously or intramuscularly have near 100% bioavailability.15 They enter the systemic circulation immediately.
- Direct Organ Targeting: Once in the blood, metals are distributed to critical organs—the kidneys, brain, and heart—before the liver has a chance to sequester them.
Lead contamination in peptides typically originates from low-quality water, reagents, or deteriorating metal equipment in unregulated labs.
- Kinetics: Upon injection, lead binds rapidly to red blood cells (99% partition).16 It has a half-life in the blood of approximately 28 to 36 days. However, this is only the initial phase. Lead mimics calcium and is eventually deposited in the skeletal system.
- The “Bone Sink”: The half-life of lead in bone is measured in decades.16 A user who injects lead-contaminated peptides for a “cycle” of a few months is depositing a toxic load that will leech back into their bloodstream for the rest of their life. This chronic low-level release is linked to hypertension, kidney disease, and cognitive decline.17
Arsenic acts by inhibiting ATP production, effectively starving cells of energy.
- Clearance: Inorganic arsenic has a short half-life in blood (3-4 hours) but rapidly distributes to tissue.18
- Accumulation: It accumulates in keratin-rich tissues (skin, hair, nails). Chronic exposure via injection can lead to peripheral neuropathy (nerve damage) and specific skin lesions.17 Because it clears from the blood quickly, a standard blood test might miss acute exposure, even as the metal damages tissue.
Cadmium is perhaps the most insidious threat in injectable products because the body has no effective mechanism to eliminate it.
- Nephrotoxicity: Once injected, cadmium binds to a protein called metallothionein. This complex is filtered by the kidneys but is then reabsorbed into the kidney cells, where the cadmium is released, causing oxidative damage and cell death.14
- Retention: The biological half-life of cadmium in the human kidney is 10 to 30 years.18 Every microgram injected adds to a cumulative burden that can lead to irreversible kidney failure later in life.
Full Blog post: Is your Chinese Peptides Dealer Poisoning You?
Good for Marek. Most people should probably use them. People that are willing to take more risk should know what they’re getting themselves into, and have a plan for risk mitigation.
a 2018 analytical forensics study
There are so many high purity peptides coming from China. This is only a concern if you have no clue what you’re doing.
Exactly, and with all the third-party testing going on from various purchasing groups we would know very fast if/when a vendor is selling less then acceptable product. There were few cases ion the past that product being sold was not up to the claimed specifications and it was all over the messaging boards, and most cases the vendors either refunded or reshipped. While there is always a remote possibility you’d end up with compromised product, the way the gray market has evolved (and grown substantially), in last couple years I am not too worried about quality. Of course, one has to always be vigilant and do their due diligence.
And a 2024 Study:
Test purchases were completed from 6 illegal online pharmacies with the highest number of links offering semaglutide products for sale without prescription at the lowest price range. Three injection vial purchases were delivered; none of the 3 Ozempic prefilled injection pens were received due to nondelivery e-commerce scams. All purchased vials were considered probable substandard and falsified products, as visual inspection indicated noncompliance in more than half (59%-63%) of the evaluated criteria. The semaglutide content of samples substantially exceeded labeled amounts by 28.56%-38.69%, although no peptide-like impurities were identified. The lyophilized peptide samples were devoid of viable microorganisms at the time of testing; however, endotoxin was detected in all samples with levels ranging between 2.1645 EU/mg and 8.9511 EU/mg. Furthermore, the measured semaglutide purity was significantly low, ranging between 7.7% and 14.37% and deviating from the 99% claimed on product labels by manufacturers.
Of course, probably not representative of the best ranked suppliers, but it does show what is out there and being sold…
And my concern is that even the testing groups out there don’t check very often for:
- Trifluoroacetic Acid (TFA)
- Endotoxins (Lipopolysaccharides)
- Dimerization and Aggregation
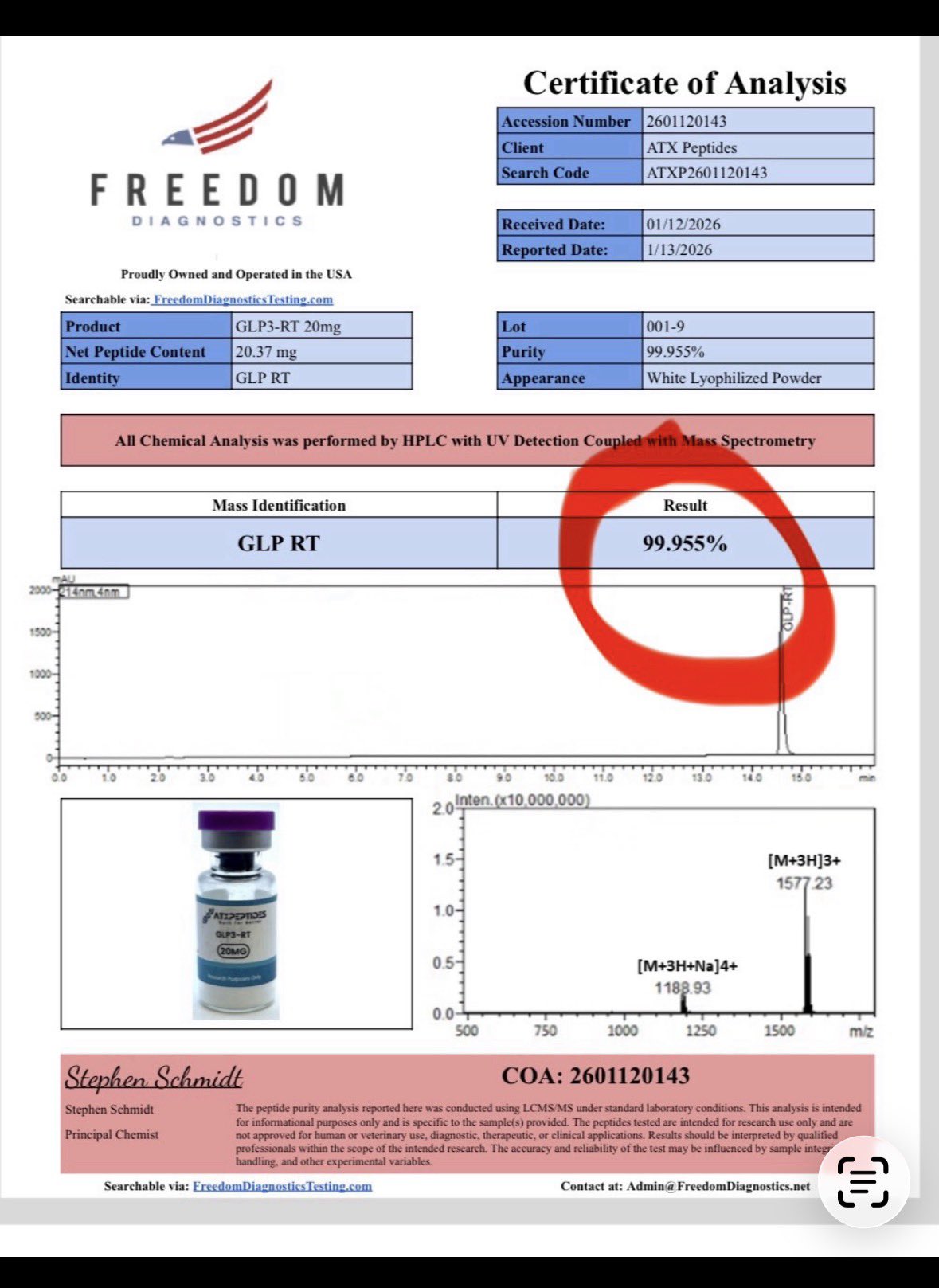
It’s a tough call to make. As @LukeMV said, you can get a COA showing 99.955 %purity. But that LC-UV-MS test is only detecting peptides and other organic molecules (basically anything that can absorb at 220nm). That LC-UV-MS test does NOT detect any metals like Pb or Cd nor does it detect endotoxin.
Also, a peptide with the same amino acids but in a slightly different order (scrambled) would elute in the same LC peak and be detected as the desired peptide but likely not have the desired biological activity (so it looks like high purity but is not).
In the end, life has risks and we all perform risk assessments every day (“can I fit my car between those two?? I"m in a hurry…”). Just recognize that by using grey market peptides vs FDA approved ones, you are taking on more risk.
How often would a scrambled peptide elute at the exact same time as an original one? 50% of the time, 10% of the time, 1% of the time?
Interestingly that is not a straight forward assessment of risk vs harm.
Risk and harm are two very different things.
While total EU/mg is important in determining risk, harm requires taking into account how the product is administered and at what dose per Kg of body weight, per hour, with the EU level in question.
64kg human (like myself) is indicated as tolerating 5 EU/kg = 320 EU total PER HOUR
- Using a mid range number from the above test example = 5.2 EU per mg
- If I inject 2.4 mg of the “black market” Ozempic peptide with that number, I’m exposed to 13 EU in one dose.
- 2.4mg is the max dose used as per the mfg, once per week
If we use the worst case exposure - 8.9511 EU/mg x 2.4mg = 21.48264 EU
still significantly below the upper limit, PER HOUR.
Both mid range and worst case findings are significantly below the upper risk level.
Practical summary for “safe” levels
For human drugs, a level is considered “safe” if the total patient exposure per dose per hour does not exceed:
- 5 EU/kg for most parenteral routes.
- 0.2 EU/kg for intrathecal/epidural routes.
You translate these exposure caps into EU/mL, EU/mg, or EU/Unit using the maximum dose (M) via K/M and then set your release/specification criteria accordingly
what are safe endotoxin levels in drugs.pdf (324.7 KB)