Meanwhile… coverage is expanding to Europe on the peptide boom, here is a new story from the Financial Times in the UK (FT.com). Its great to finally see something written where the organization (the FT in this case) actually has reporters on the ground in China going around and looking to talk with the companies, find their facilities, and talk with the resellers in China. Related threads: NYT: Chinese Peptides are Latest Biohacking Trend in Tech Industry
Just to be clear… if you are buying from China you probably have no idea who the actual manufacturer is, what the purity or contaminants might be, and zero guarantees or recourse if something goes wrong… It’s not much different than buying drugs on a street corner. And this isn’t just a problem with Peptides… it’s with every chemical or drug you might want to buy via a middleman in China. They all “say” they are the manufacturer…
‘I wouldn’t dare take these drugs’: how China supplies untested peptides to the west
In nondescript office blocks across China, salespeople are pitching injectable drugs to overseas customers, fuelling a fast-growing black market in peptides — compounds that online influencers claim can improve everything from sleep and memory to skin elasticity.
The surge has been driven partly by the runaway success of GLP-1 weight-loss drugs such as semaglutide and tirzepatide, which belong to a broader class of peptide-based therapies.
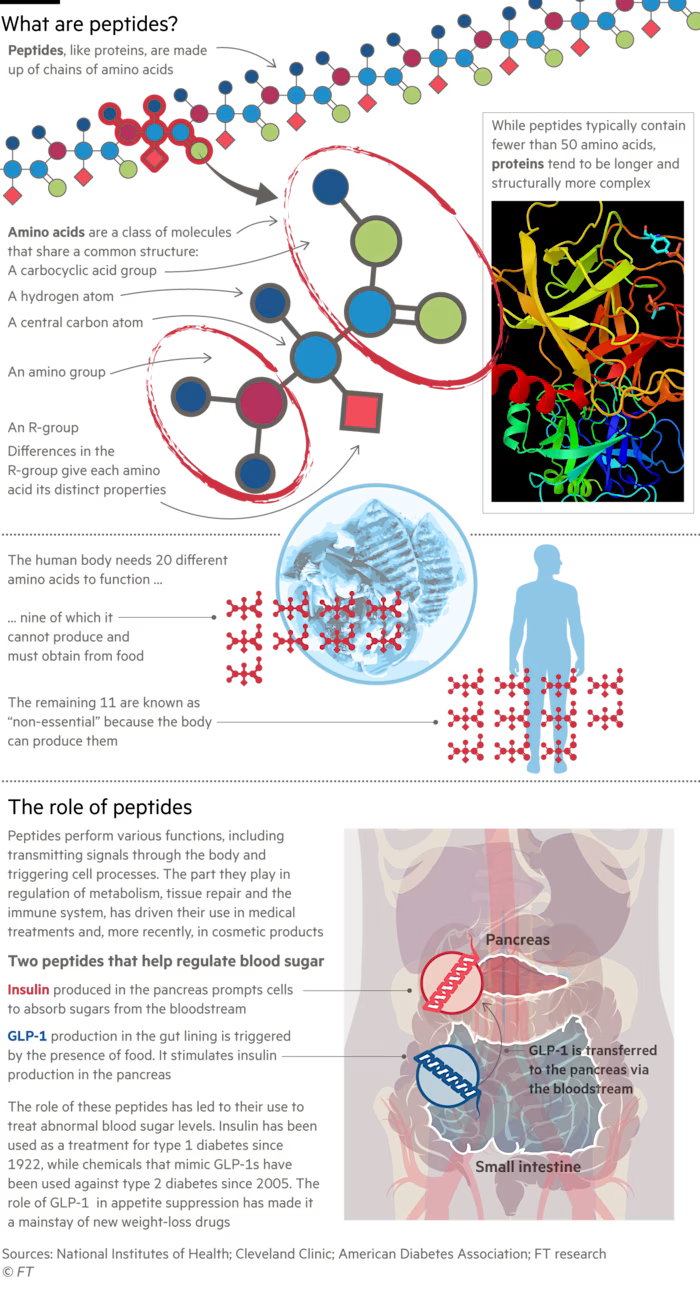
Peptides are short chains of amino acids that act as signalling molecules in the body, influencing hormonal and metabolic processes. Advocates claim they could one day offer better health with fewer side effects than conventional pharmaceuticals.
But many of the compounds circulating online are either banned for human use by regulators — including in the US — or remain prohibitively expensive through legitimate medical channels. That has pushed demand towards online sellers, many of them based in China.
Sensing an opportunity, hundreds of Chinese traders have established operations exporting low-cost peptides to western buyers, according to interviews with multiple sellers and an FT review of online marketplaces. What has emerged is a highly secretive, unregulated shadow economy.
“I used to sell paper, but business was bad because of the weak economy,” said one seller, who asked to remain anonymous because selling unprescribed peptides for human use is illegal in both China and the US. “I heard from friends that there are people in Shenzhen selling peptides overseas who made tons of money.”
…
Industry insiders estimate there are about 1,000 Chinese sellers targeting overseas customers, a figure that has climbed sharply in recent months. Rising competition has pushed prices down. Sellers quoted prices such as $65 for 10 vials of BPC-157 at 10mg, or $70 for the same quantity of semaglutide — which compares to $110 and $1,000 for the same quantities on US websites.
Despite the proliferation of sellers, industry insiders said the underlying supply chain was far more concentrated. Most peptides are produced by about a dozen factories clustered in Shenzhen and Changsha, the capital of Hunan province. These facilities originally manufactured active pharmaceutical ingredients for the pharmaceutical industry before pivoting towards the grey market. Multiple layers of intermediaries now sit between factories and consumers.
“We never touch the product. We don’t know who makes it,” said one seller. That opacity is deliberate, even as traders circulate videos purporting to show vials on production lines, creating the impression that buyers are dealing directly with manufacturers.
Finding sellers online is easy. They advertise openly on cross-border ecommerce platforms such as Global Sources, as well as on Facebook, Telegram and WhatsApp. Tracking their physical presence is far harder. The FT visited the registered addresses of eight suppliers and found that most used false locations, with no functioning phone numbers or email contacts.
Chinese authorities have been cracking down on domestic sales of unregulated GLP-1 weight-loss drugs, arresting scores of sellers. A review of court records shows at least 40 cases of people being charged with black-market peptide sales. Penalties can include fines of up to 10 times the revenue earned. As a result, peptides popular in the US are all but unavailable to Chinese consumers.
Inside one office in China visited by the FT, a group of young women chatted with customers in Brazil, the US and Canada. They used ChatGPT to draft sales copy for WhatsApp messages and worked with western influencers who promote the products on TikTok and Facebook in exchange for commissions.
Medical experts warn of the dangers of sourcing drugs from unregulated suppliers. While sellers often provide lab reports claiming near-100 per cent purity, such tests do not typically screen for contaminants such as heavy metals or verify handling and storage conditions.
“If you’re buying peptides online without a prescription, you don’t know whether there are issues with purity, contamination or transport,” said Dr Mohammad Enayat, who runs a longevity clinic in London and prescribes peptides sourced through regulated channels.
Back in China, one seller said he had no intention of trying the products himself. “I’m overweight, but I wouldn’t dare take these drugs,” he said, laughing. “It’s westerners who are obsessed with them. I just sell them.”
Read the full story: ‘I wouldn’t dare take these drugs’: how China supplies untested peptides to the west (FT)