Perhaps verteporfin+microneedling (or other therapy that stimulates wound healing like laser treatment) could rejuvenate elastin. Some hair transplant surgeons have already been using verteporfin as it shows some ability to regenerate excised hair follicles, in addition to reducing scarring.
The Longaker lab at Stanford has done much of the work on how verteporfin prevents scarring. If you have time, great recent lecture (with health implications for more than just skin regeneration):
In pigs (which have skin closer to humans) verteporfin can normalize the elastic ratio in response to a wound, which suggests that it regenerates elastin (which normally is poorly regenerated in adult wound healing [ref]).
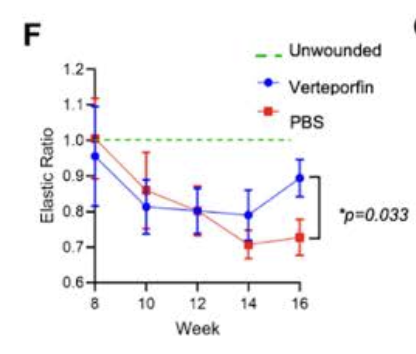
Data from Elucidating the Role of Tissue Mechanics in Wound Repair and Regeneration (PhD thesis)
Verteporfin is already an approved photosensitizing drug (it generates ROS in response to certain light frequencies) for macular degeneration, but in wound healing it acts by inhibiting the YAP pathway. This prevents Engrailed-1+ fibroblast conversion, which would otherwise promote a scarring/fibrotic response.
Also of note, ELNs promoter is structured like a housekeeping gene [ref] (these are constitutively expressed genes—e.g the glycolytic enzyme GAPDH—that are necessary for basic cellular life, regardless of cell type), and it seems that the drop-off in tropoelastin synthesis during adulthood is due to post-transcriptional regulation. In particular, RNA interference from the miR29 family of micro RNA’s seems to play an important role, and miR29 antagonism can raise tropoelastin protein levels in adult human dermal fibroblasts, relative to controls. [ref].
It’s worth mentioning TGF-β, which is the ‘master regulator’ of fibrosis, and that miR29 family downregulation is a hallmark of TGF-β signaling, where it acts to increase translation of extracellular matrix proteins. [ref] So although miR29 antagonism might upregulate tropoelastin synthesis, by promoting collagen synthesis it would be expected to drive a fibrotic phenotype.
Not that you would want to upregulate TGF-β anyways, as its primary transcriptional response is the pro-aging cytokine IL-11, which (at least in certain tissues) is necessary for the TGF-β-mediated fibrotic response. [ref] I’m working on a separate post to highlight the fact that rapamycin activates TGF-β signaling, and that this very well might cap any associated increase in health/lifespan, especially with high doses and/or non-intermittent usage.