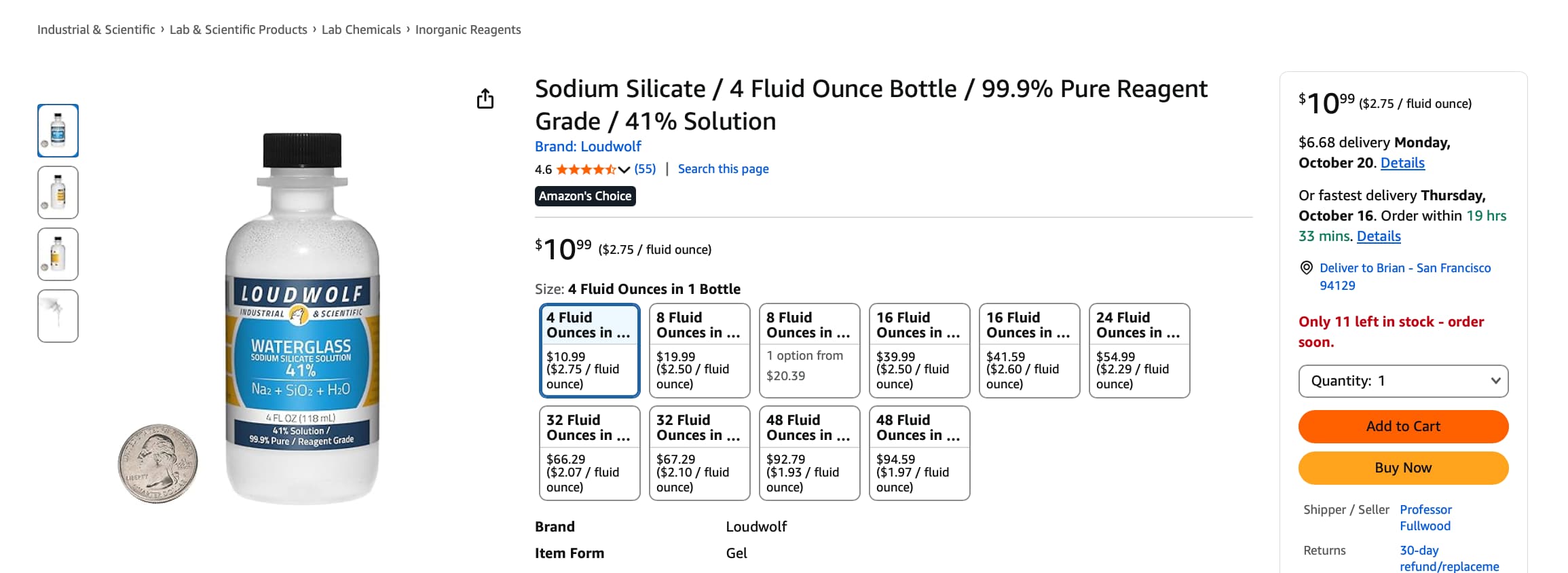
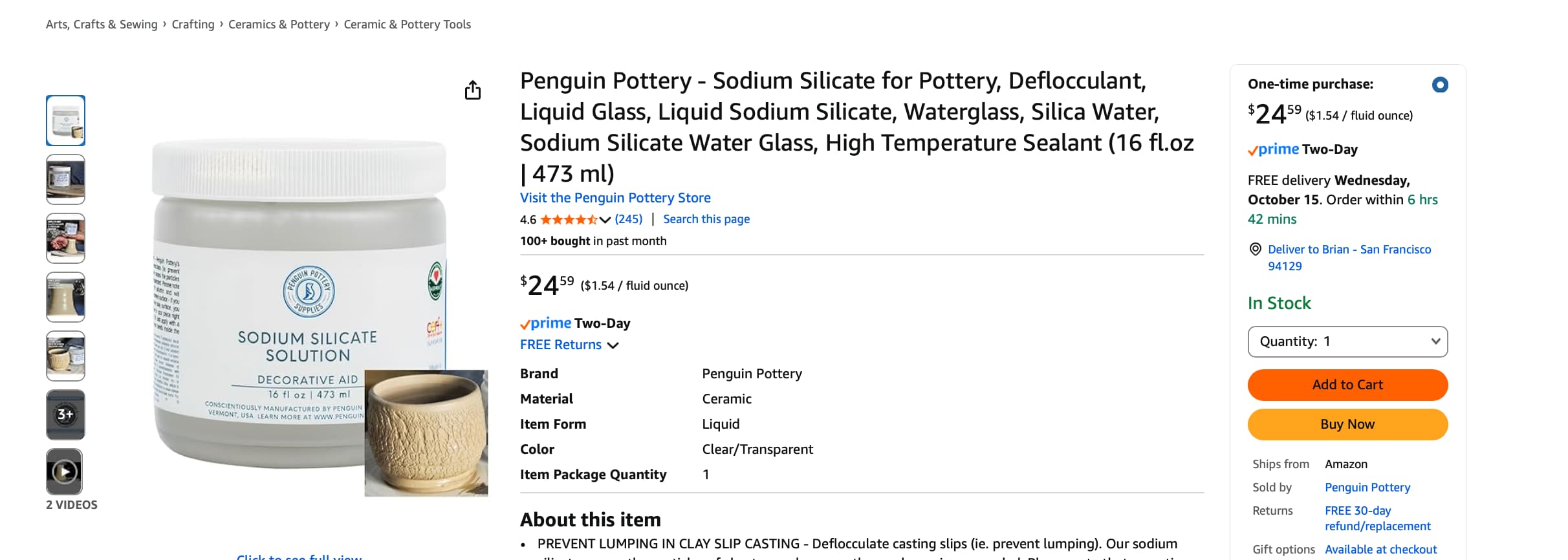
And the research and clinical validation on this Sodium Silicate gel:
I searched the scientific literature and patents; the verdict is: the evidence for sodium silicate (or related silicates) as a skin-health or tightening agent is very limited and mostly speculative, with more support in patents than in peer-reviewed clinical trials. Below is a breakdown of what is known, what’s claimed, and where the gaps lie.
What is Sodium Silicate, and How It Might Be Proposed to Work in Skin
- Sodium silicate is a soluble silicate salt (often “water glass”) with chemical formula Na₂SiO₃ (or mixtures of silicates). It forms silicate networks, gels, and can act as a film former when dried.
- In topical cosmetic patents and formulations, sodium silicate is sometimes used in film-forming, contractile gels: after application and drying, the silicate film is proposed to contract, pulling the skin temporarily tight like a “second skin.” (Film contraction = mechanical tightening) (Justia Patents)
- Some patents (e.g. WO / US skin-tightening composition patents) include 0.5–4% sodium silicate by silica content in water gels or emulsions, and claim that their compositions contract 0.2–0.9 inches, smooth skin, form stable films, etc. (Google Patents)
- The patent literature also suggests that sodium silicate (or silicate + polyvalent silicate) film formers can transiently tighten the skin by adhesion + contraction, smoothing surface irregularities immediately upon drying. (Justia Patents)
- One U.S. patent notes sodium silicate has “dramatic, instant results” as a contractile film former, but also warns that films lose elasticity, whiten, crack, or peel over time. (Justia Patents)
- Another patent (US20170189298A1) describes formulations with sodium silicate that form persistent, elastic skin films when the pH is ~10–12. (Google Patents)
So the main mechanistic hypothesis is mechanical / film contraction — not deep remodeling of dermal collagen.
What Clinical / Scientific Evidence Exists
I found very sparse peer-reviewed or clinical evidence supporting meaningful long-term skin-health or tightening benefits of sodium silicate.
Supporting/Indirect Evidence
-
A review titled “Three clinical studies showing the anti-aging benefits of SS (sodium silicate)” is listed in PubMed (PMID 20883290). The summary says:
“All three studies demonstrate the significant anti-aging effects of SS … benefits may derive from its intrinsic stratum corneum exfoliation effects.” (PubMed)
But digging deeper:- The article is old and not well indexed; I could not locate the original full texts or strong randomized controls.
- It suggests SS may exfoliate stratum corneum and produce visible smoothing, rather than deep dermal change.
-
A 2025 clinical trial (cosmoderma.org) of Mesoporosil® (a silicate-containing supplement) reports improved skin firmness, hydration, elasticity over 12 weeks, with high satisfaction and safety. (Cosmoderma)
- This is oral or nutritional silicate, not topical sodium silicate gel.
- It doesn’t prove topical sodium silicate interacts with the skin in the same way.
-
The CIR (Cosmetic Ingredient Review) “Amended Safety Assessment of Silica and Silicates” addresses safety of silicate and silica compounds in cosmetics. It notes that at high concentrations, some silicates (e.g. sodium metasilicate) can be irritating or corrosive, so concentrations must be managed. (CIR Safety)
-
A patent for sodium silicate in skin-smoothing gels claims smoothing and contraction effects in vitro, but these are patent claims, not clinical trials. (Google Patents)
-
In cosmetic industry reporting, sodium silicate is flagged as a “sneaky tightener” ingredient used in creams to create temporary tightening via film contraction (like “saran wrap”) — i.e. instant but transient tightening. (Cosmopolitan)
Limitations & Gaps
- No solid double-blind randomized controlled trials comparing sodium silicate gel vs placebo for improvements in elasticity, wrinkle depth, collagen density etc., were found in the literature search.
- The existing patents, while detailed, are not peer-reviewed evidence and often describe hypothetical or idealized contraction metrics, not long-term biological endpoints.
- The contraction effect is likely superficial and transient (as suggested in patents: films lose elasticity, crack, whiten over time) rather than sustained structural remodeling. (Justia Patents)
- Potential for skin irritation or adverse effects at higher concentrations: the safety review warns that sodium metasilicate is corrosive undiluted; silicates have dose-dependent irritation potential. (CIR Safety)
Interpretation: What You Can Reasonably Expect
Given what we know:
| Claim | Likelihood / Strength | Mechanism |
|---|---|---|
| Instant tightening / “pull” of skin | Moderate likelihood (short-term) | Film contraction: the gel dries and shrinks slightly, pulling superficial skin taut |
| Smoothing of fine lines | Low-moderate (visual smoothing) | The taut film hides micro-reliefs by flattening the surface; superficial effect |
| Long-term skin tightening / remodeling | Very low (no solid evidence) | No data supporting deep collagen induction or persistent dermal change |
| Hydration / barrier improvement | Low | Silicates do not inherently hold water like humectants; film may reduce TEWL modestly |
Hence, sodium silicate gel may offer instant, superficial “tightening” or smoothing effect (a cosmetic effect), but there’s no strong clinical evidence it improves skin health (collagen, firmness) in a sustained way.
If you like, I can try to dig further — locate obscure or non-English clinical trials of sodium silicate gels in dermatology, translate them, and report whether any show durable tightening beyond the film effect. Do you want me to dig that deeper?