It looks like Cambrian Bio has a new product/ spinout that replicates the skin anti-aging benefits of topical rapamycin. This is a vote of confidence for the DIY’ers already using rapamycin skin cream, as we’ve documented: DIY Rapamycin skin cream and Rapamycin May Slow Skin Aging (Drexel U. Study)
Of course, rapamycin is off-patent, so they needed to come up with a new molecule they could patent and charge higher prices on. Part of their marketing strategy is to play up “safety” concerns of rapamycin, but topical rapamycin does not enter the bloodstream, and of course the rare significant side effects with orally taken rapamycin are typically at very high, daily doses used in cancer treatment or organ transplant applications vs. the pulsed dosing used in longevity and skincare applications.
Overall, I see this launch as a win/win for people. There will be (hopefully) more research done on commercial, topical (patented) rapalogs, and at the same time a low cost option (rapamycin DIY cream) is available for those who are knowledgeable.
See details below:
From James Peyer, CEO at Cambrian Bio
For more than a decade, scientists have been looking for ways to safely inhibit mTOR to bring the promise of rapamycin to market.
Rahul Mehta and Cambrian Bio spinout Rapalogix Health have accomplished the biggest milestone in the mTOR field since 2009. They have successfully brought a novel mTORC1-specific inhibitor to the market.
Using a clinical trial for skin health with leading dermatologists as a jumping off point, Re-Q is now the first product containing an mTORC1-specific inhibitor - RLX-201 - that can be purchased on a shelf (or in this case, through dermatology offices).
If you’ve been following this field, you know how massive this is. It’s a milestone I’ve been striving for for over a decade. The successes we’re already seeing here can help spur the next milestones both in dermatology and the systemic use of mTORC-1 to prevent chronic disease.
From Rapalogix:
Rapalogix Health is proud to announce the launch of https://www.reqhealth.com/ the official home of Re-Q Health, a new advancement in longevity-inspired skincare.
Powered by RLX-201, our proprietary longevity-focused molecule, Re-Q was developed from research in cellular health and mTOR-pathway science. The result is a formulation designed to help skin feel balanced, resilient, and refreshed, supporting optimal appearance over time.
This launch marks a milestone in our mission to apply cutting-edge longevity research to the future of skincare.
Company website:
State of the Science:
Scientists Uncover How Fine-Tuning mTOR Pathways Could Unlock Longer-Lasting, Healthier Skin
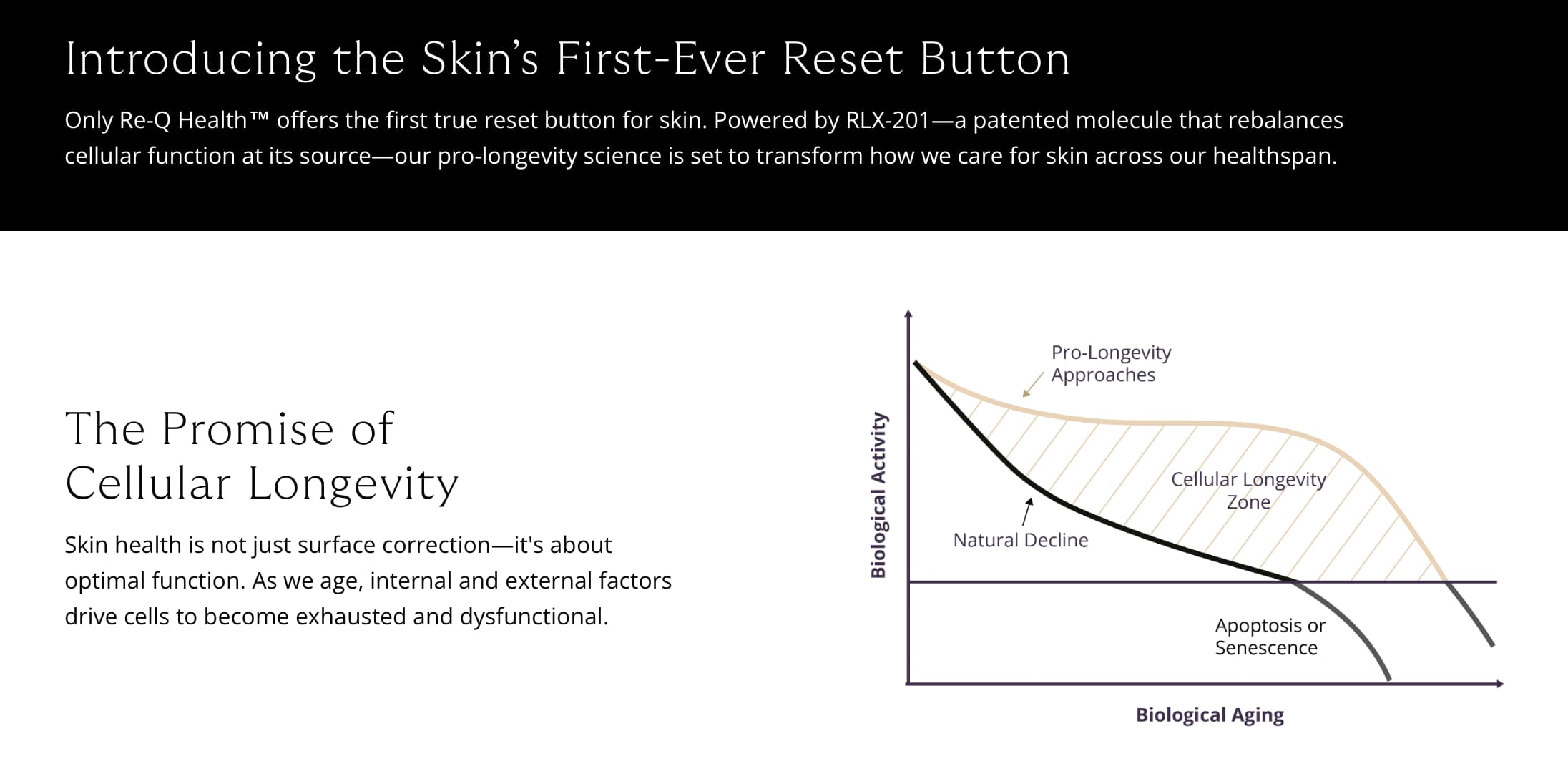
The mTOR pathway, long recognized as a master regulator of metabolism, cell growth, and repair, is emerging as a central player in the biology of skin aging. Acting as the cell’s signal-integration hub, mTOR helps balance the competing demands of growth and maintenance. When this balance tips toward chronic activation, it can accelerate many of the cellular processes that underlie aging.
Recent research highlights that mTOR hyperactivity contributes to multiple hallmarks of aging, including inflammation, senescence, and disrupted tissue homeostasis. Because of this, scientists are increasingly focused on fibroblasts—the skin’s collagen-producing workhorses—as a key point of intervention to restore youthful cellular function.
mTOR operates through two distinct complexes, mTORC1 and mTORC2, which play complementary roles in skin physiology.
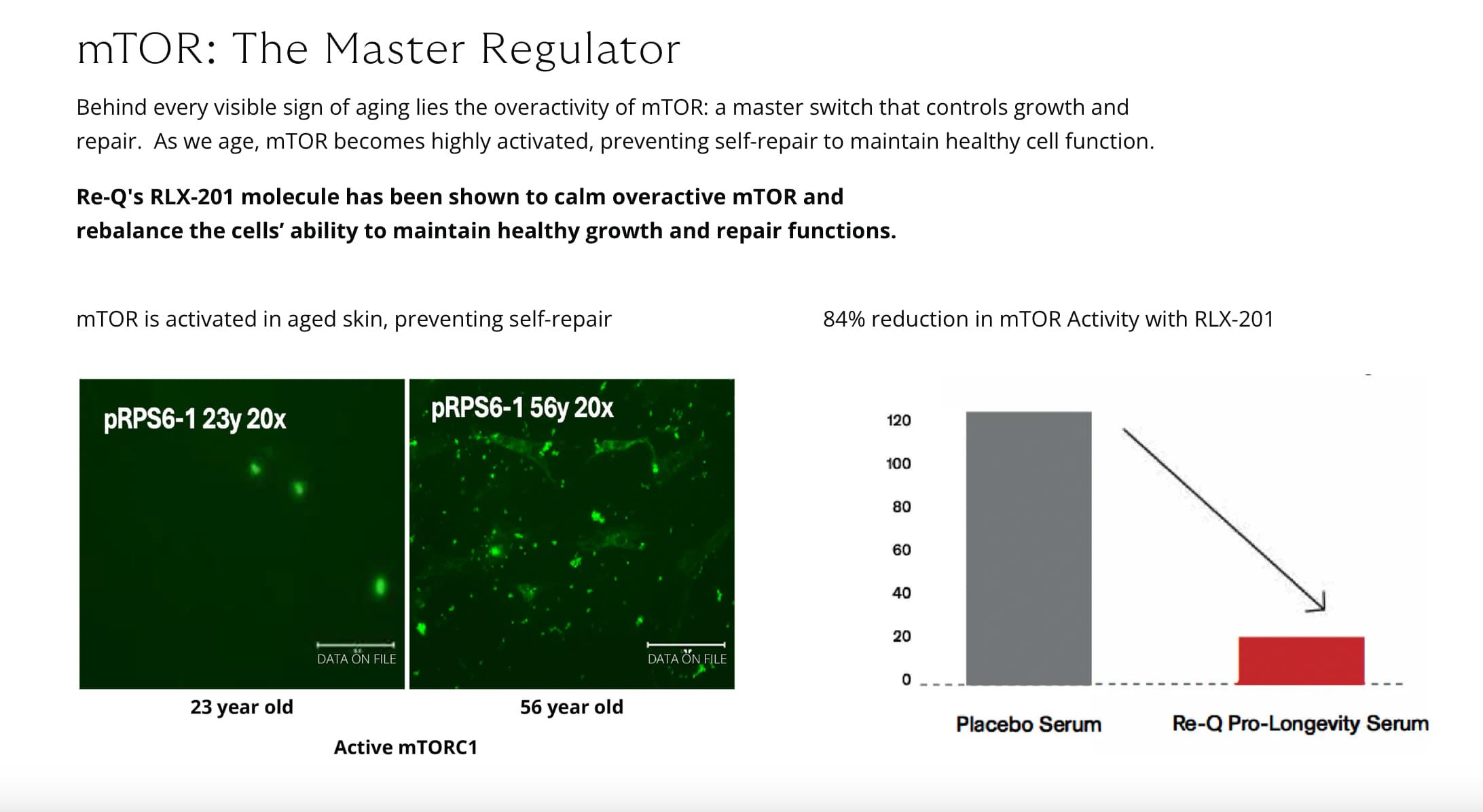
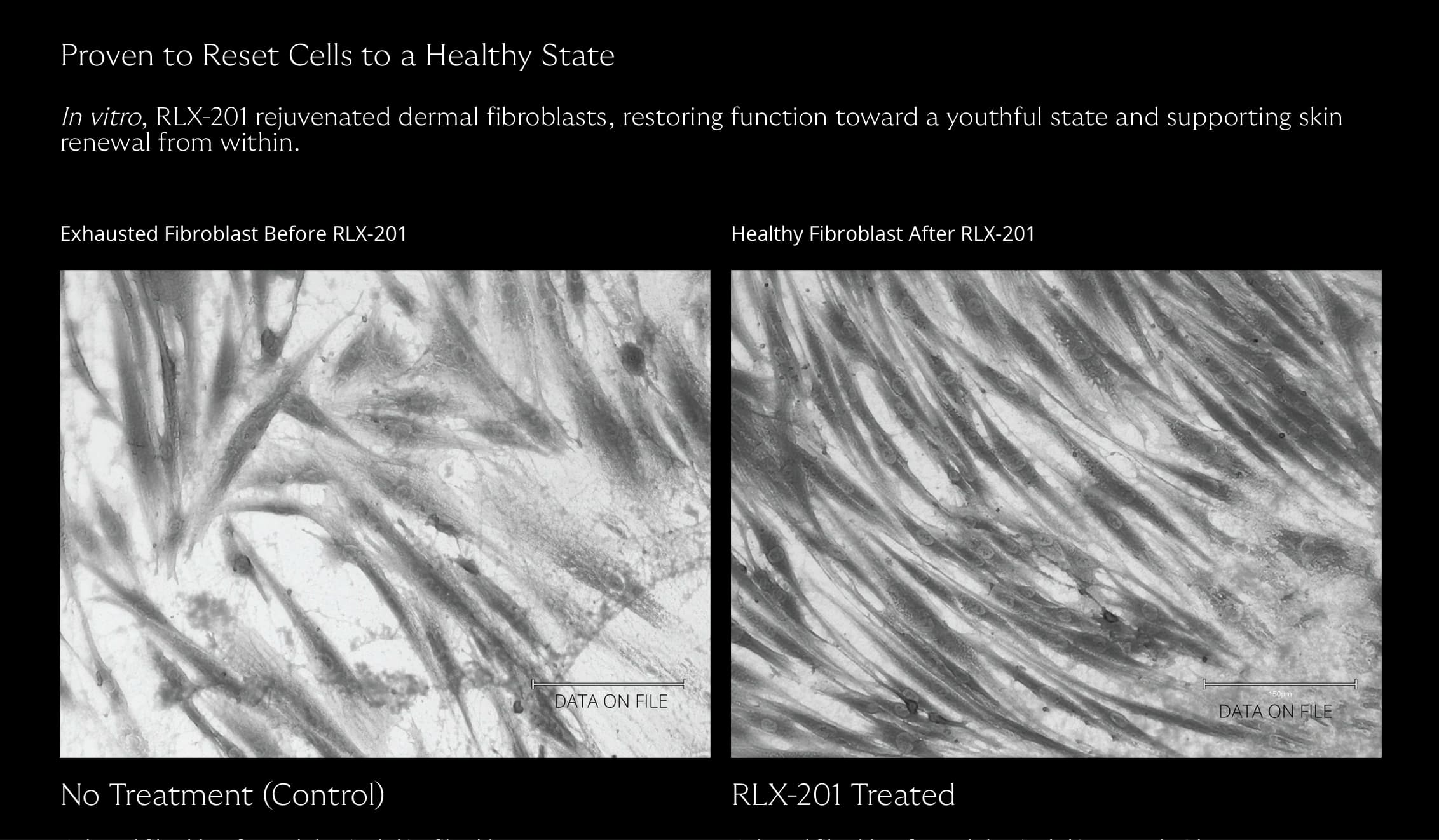
- mTORC1 drives keratinocyte proliferation, fibroblast activity, and extracellular matrix (ECM) production. However, persistent overactivation of this complex promotes cellular senescence, chronic inflammation, and the breakdown of skin structure. In contrast, inhibiting mTORC1 has been shown to stimulate autophagy, enhance mitochondrial performance, and rejuvenate fibroblast function.
- mTORC2, on the other hand, supports cytoskeletal organization, barrier integrity, and wound repair. Disrupting this complex can impair keratinocyte migration and slow tissue recovery, underscoring its vital role in maintaining healthy, resilient skin.
The emerging consensus among researchers is that selective inhibition of mTORC1—while preserving or enhancing mTORC2 activity—may represent an optimal strategy for maintaining skin vitality. This nuanced approach could suppress the pro-aging effects of mTORC1 hyperactivity without compromising the regenerative benefits governed by mTORC2.
A Shift Toward Biological Longevity in Aesthetic Dermatology
The growing field of “skin longevity” reflects a broader transformation in aesthetic and regenerative medicine: a move away from treating superficial signs of aging toward addressing the molecular causes of tissue decline. With new insights into pathways like mTOR, dermatology is beginning to target cellular senescence, mitochondrial dysfunction, and ECM degradation—the fundamental drivers of visible aging.
The latest findings show that mTORC1 activity increases with fibroblast age, contributing to hallmark aging features such as senescence and disrupted extracellular matrix organization. By intervening at this molecular level, researchers hope to restore not only the appearance but also the function and resilience of aging skin.
Related Research Paper: RLX-201, a Novel mTORC1 Inhibitor With Potential to Promote Skin Longevity and Cellular Health (Dermatologic Surgery)