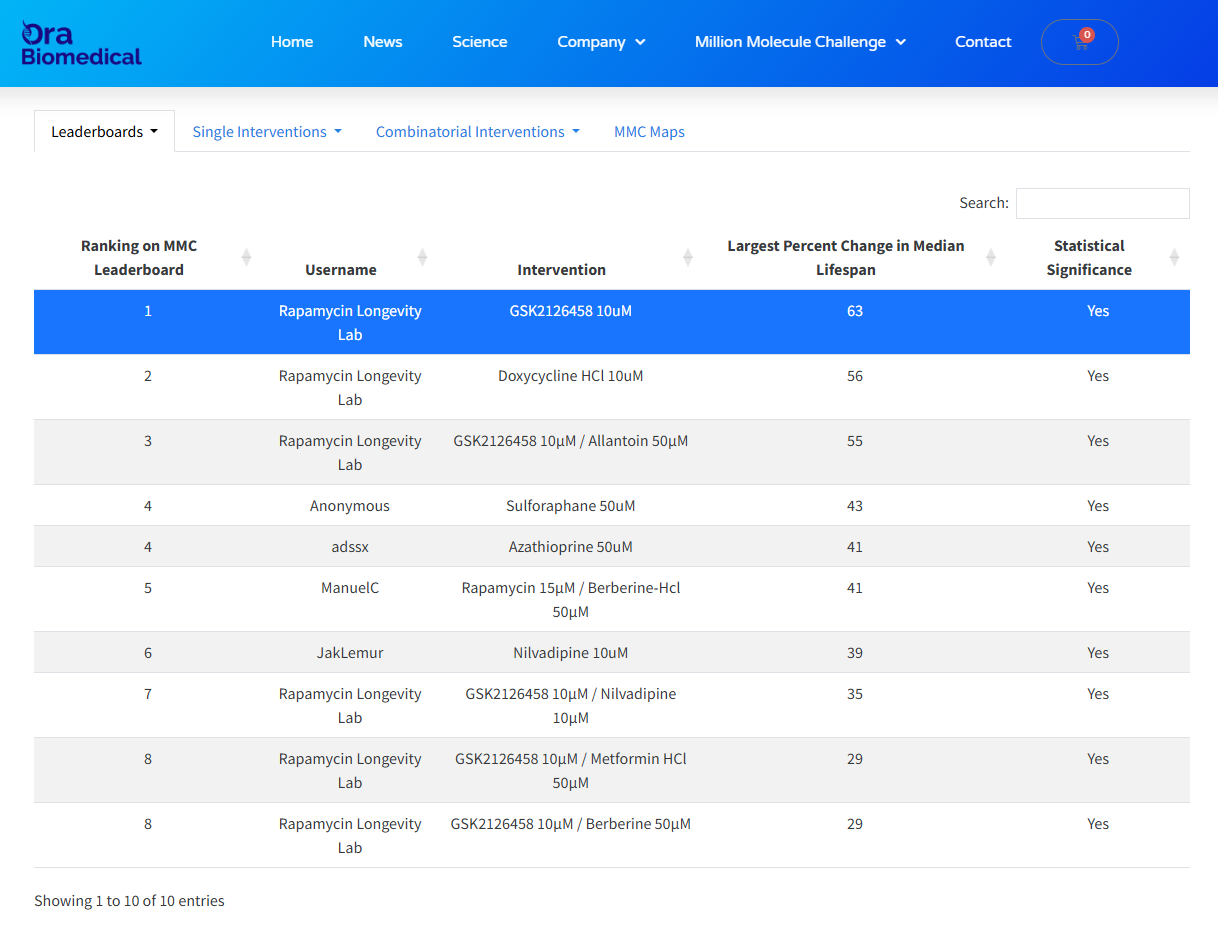
It looks interesting with a shorter half life. However, what I like about Rapamycin is that it has had a lot of use and therefore the safety profile is quite clear.
https://publications.ersnet.org/content/erj/53/3/1801992
Abstract
Phosphatidylinositol 3-kinases (PI3Ks) and mammalian target of rapamycin (mTOR) play a role in the pathogenesis of idiopathic pulmonary fibrosis (IPF). Omipalisib (GSK2126458) is a potent inhibitor of PI3K/mTOR.
A randomised, placebo-controlled, double-blind, repeat dose escalation, experimental medicine study of omipalisib in subjects with IPF was conducted (NCT01725139) to test safety, tolerability, pharmacokinetics and pharmacodynamics. Omipalisib was dosed at 0.25 mg, 1 mg and 2 mg twice daily for 8 days in four cohorts of four subjects randomised 3:1 to receive omipalisib or placebo (two cohorts received 2 mg twice daily).
17 subjects with IPF were enrolled. The most common adverse event was diarrhoea, which was reported by four participants. Dose-related increases in insulin and glucose were observed. Pharmacokinetic analysis demonstrated that exposure in the blood predicts lung exposure. Exposure-dependent inhibition of phosphatidylinositol 3,4,5 trisphosphate and pAKT confirmed target engagement in blood and lungs. 18F-2-fluoro-2-deoxy-d-glucose(FDG)-positron emission tomography/computed tomography scans revealed an exposure-dependent reduction in 18F-FDG uptake in fibrotic areas of the lung, as measured by target-to-background, ratio thus confirming pharmacodynamic activity.
This experimental medicine study demonstrates acceptable tolerability of omipalisib in subjects with IPF at exposures for which target engagement was confirmed both systemically and in the lungs.
https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/omipalisib
what research is there as to the side effects of omipalisib
ChatGPT said:
Omipalisib (GSK2126458) is a dual inhibitor targeting the PI3K/mTOR signaling pathways, currently under investigation for its potential in treating various cancers and idiopathic pulmonary fibrosis (IPF). Clinical and preclinical studies have identified several side effects associated with its use:PubMed+3PMC+3ASH Publications+3
Common Side Effects
-
Gastrointestinal Issues: Diarrhea is frequently reported. In a Phase I cancer trial, 8% of patients experienced grade ≥3 diarrhea, and in an IPF study, it was the most common adverse event .PubMed
-
Skin Reactions: Rashes occurred in up to 74% of patients in some studies, with 5% experiencing severe cases .EMBL-EBI homepage+1PubMed+1
-
Fatigue: Reported in approximately 21% of patients .
Metabolic Effects
-
Insulin Resistance and Hyperglycemia: Omipalisib has been associated with dose-related increases in insulin and glucose levels, indicating potential insulin resistance .UCL Discovery+1ERS Publications+1
Cardiac Effects
-
QT Interval Prolongation: Preclinical studies in dogs showed that omipalisib prolonged the QT interval and, under certain conditions, led to arrhythmias like Torsade de Pointes in 20% of subjects .PMC
Tolerability
While omipalisib has demonstrated pharmacodynamic activity, its tolerability varies. In some studies, 42% of patients required dose interruptions due to adverse events . However, short-term dosing in IPF patients showed acceptable tolerability .UCL Discovery+1gsk-studyregister.com+1EMBL-EBI homepage
Conclusion
Omipalisib’s side effect profile includes gastrointestinal disturbances, skin reactions, fatigue, metabolic changes, and potential cardiac effects. These findings underscore the need for careful monitoring and further research to fully understand its safety and efficacy.
My Conclusion
It strikes me that there is limited research with humans and what appear to be worse side effects.