Part 2: The Biohacker Analysis
Study Design Specifications
-
Type: In vivo (murine intervention) and In vitro/Ex vivo (Human Single-Nucleus Multiomics Atlas).
-
Subjects:
-
Animal: Aged C57BL/6 mice (18 months old, approx. 56 human years).
-
Human: Skeletal muscle biopsies from young (19-27y) vs. aged (60-77y) donors for atlas generation.
-
N-number: Mice n=8 per group; Human donors n=10.
-
Lifespan Data: Not Reported. The study focused exclusively on healthspan metrics (sarcopenia reversal) over a 3-month treatment window. No median or maximum lifespan curves were generated.
Mechanistic Deep Dive
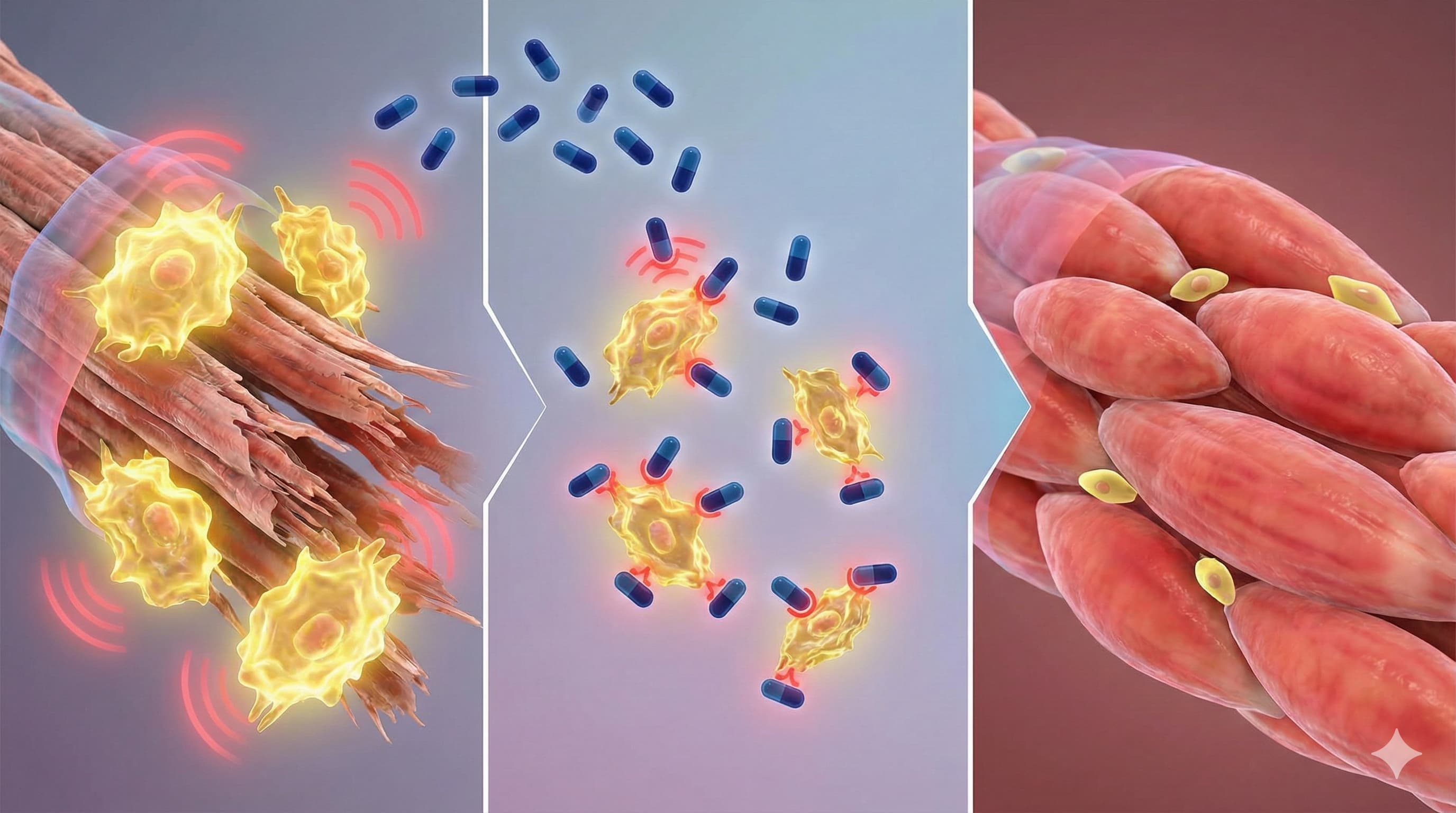
The study identifies the CCL5-CCR5 axis as a critical driver of the senescence-associated secretory phenotype (SASP) in skeletal muscle.
-
Pathway: Senescent cells in aged muscle secrete high levels of CCL5, which binds to CCR5 receptors on neighboring cells (paracrine) and the senescent cells themselves (autocrine).
-
Downstream Effect: This binding activates NF-κB and MAPK pathways, perpetuating inflammation and locking muscle stem cells (MuSCs) in a dysfunctional state.
-
Intervention: Maraviroc (a CCR5 antagonist) breaks this feedback loop. Unlike senolytics (e.g., Dasatinib + Quercetin) which kill senescent cells, Maraviroc acts as a senomorphic—it suppresses the toxic SASP phenotype without necessarily killing the cell, effectively “muting” the zombie cells.
-
Organ Priority: Skeletal Muscle (specifically improving MuSC regenerative capacity).
Novelty
-
First Human Senescence Atlas: Creates a high-resolution map of exactly which cell types become senescent in human muscle (identifying heterogeneity previously missed).
-
Drug Repurposing: Validates an FDA-approved HIV entry inhibitor as a potent anti-sarcopenia agent, shifting the focus from “killing” senescent cells to interfering with their intercellular signaling.
Critical Limitations
-
Route of Administration: The mice received intraperitoneal (i.p.) injections. Maraviroc has variable oral bioavailability in humans (~23-33%), so translational efficacy via oral dosing remains to be confirmed for this specific indication.
-
Short Duration: The treatment lasted only 3 months. Long-term effects of chronic CCR5 inhibition in non-HIV populations (e.g., immune surveillance risks) are not fully characterized in the context of aging.
-
No Lifespan Data: We don’t know if this healthspan improvement translates to extended life.
-
Missing Data: The study did not report effects on other organs (liver, kidney) where CCR5 might play a different role in aging.
Part 3: Actionable Intelligence
The Translational Protocol
-
Human Equivalent Dose (HED):
-
Animal Dose: 10 mg/kg (administered every 2 days).
-
Math: 10 mg/kg×(3/37)≈0.81 mg/kg.
-
Human Result: For a 70 kg human, this is ~57 mg (every 2 days) or roughly 30 mg/day.
-
Note: The standard clinical dose for HIV is 300 mg BID (600 mg/day). The longevity/sarcopenia effective dose appears to be ~1/20th of the antiviral dose, suggesting a wide therapeutic window.
-
Pharmacokinetics (PK/PD):
-
Bioavailability: ~23–33% (Oral). Increases significantly with high-fat meals (reduced absorption) or strong CYP3A inhibitors (increased exposure).
-
Half-life: ~14–18 hours. Daily dosing is sufficient to maintain blockade.
-
Safety & Toxicity Check:
-
Safety Profile: Generally well-tolerated.
-
NOAEL (Mice): 200 mg/kg/day (significantly higher than the 10 mg/kg efficacy dose).
-
Black Box/Warnings: Hepatotoxicity (liver toxicity) has been reported. Severe hypersensitivity reactions (SJS/TEN) are rare but possible.
-
Interactions: Major CYP3A4 substrate.
-
Contraindication: Do not mix with St. John’s Wort (inducer).
-
Caution: Interaction with Rapamycin (Sirolimus) is highly probable as both compete for/are metabolized by CYP3A4.
Benefits
| Metric |
Improvement |
Biological Significance |
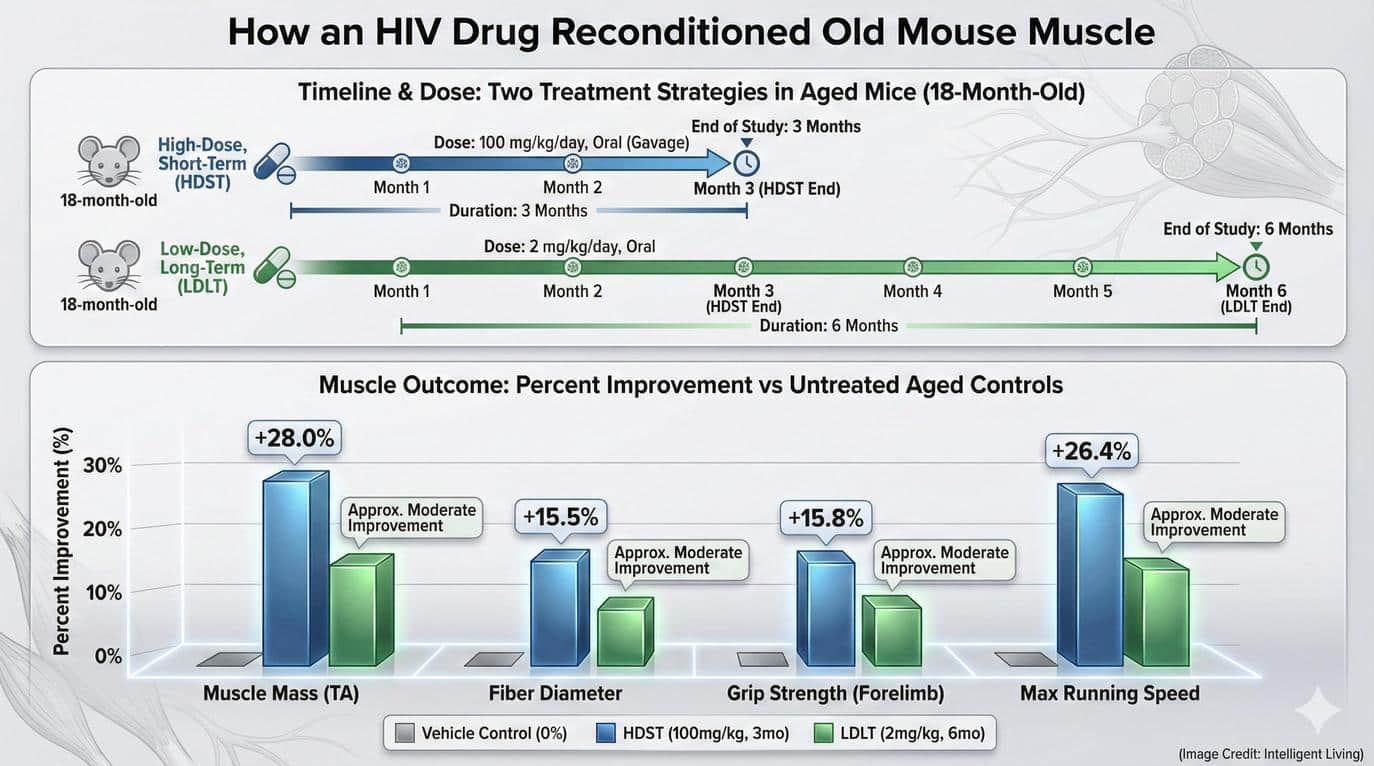
| Muscle Mass |
+28% |
High Impact: In a geriatric mouse model, a nearly 30% regain in lean mass without anabolic steroids is exceptional. This suggests the restoration of “lost” tissue, not just hypertrophy of existing fibers. |
| Fiber Diameter |
+15% |
Hypertrophy Signal: Cross-sectional area (CSA) increase indicates that the existing myofibers are repairing and protein synthesis is outpacing degradation (reversing the catabolic state of aging). |
| Grip Strength |
+16% |
Functional Quality: Mass does not always equal strength (“dynapenia” is the loss of strength). This metric confirms that the new muscle tissue is functional and contractile, not just “wet weight” or edema. |
| Endurance |
+20% |
Mitochondrial/Metabolic: Running endurance relies on energy efficiency. This gain implies that Maraviroc likely improved mitochondrial health or vascular supply (capillarization) alongside the muscle fibers. |
Biomarker Verification Panel
-
Efficacy Markers:
-
Functional: Grip strength (dynamometer), 6-minute walk test.
-
Blood: hsCRP (general inflammation), IL-6, and specifically CCL5 (RANTES) levels (though serum levels may not perfectly reflect tissue concentrations).
-
Safety Monitoring:
-
Liver Panel: ALT/AST and Bilirubin (Crucial due to hepatotoxicity risk).
-
Immune: CD4+ T-cell count (chronic CCR5 blockade can theoretically impact immune surveillance).
Feasibility & ROI
-
Sourcing: Prescription only (brand name Selzentry/Celsentri). Generic Maraviroc is available.
-
Cost:
- Generic retail (US): ~$300–$600/month.
- Cost Plus Drugs (US): ~$292/month.
- India (Off-shore/Online) Pharmacies: ~$50 per month.
- At the extrapolated “low dose” (approx 1/10th to 1/20th of HIV dose), a single month’s supply of 300mg tablets could theoretically last ~6-12 months if pill splitting were possible/stable (stability of split tablets unverified).
-
ROI: High potential for specific sarcopenia cases; lower ROI for general “prevention” due to cost and liver risk compared to exercise (the gold standard).
Population Applicability
-
Target: Individuals >60 with diagnosed sarcopenia or frailty.
-
Contraindications: Severe renal impairment (CrCl <30 mL/min) if taking CYP3A inhibitors. History of liver disease.
Part 4: The Strategic FAQ
1. Does Maraviroc extend maximum lifespan? Answer: [Confidence: Low] Data Absent. The study was a short-term (3-month) intervention focused on muscle function (healthspan). No longevity curves were generated. While improved muscle health correlates with longevity, no direct evidence exists yet for Maraviroc.
2. Can I take Maraviroc with Rapamycin? Answer: [Confidence: High] Proceed with Extreme Caution. Both drugs are metabolized by CYP3A4. Rapamycin is a substrate; Maraviroc is a substrate. Competing for the same enzyme can lead to unpredictable elevations in blood levels of both drugs, increasing the risk of Rapamycin toxicity (immunosuppression, mouth sores) or Maraviroc hepatotoxicity. Dose adjustments would be mandatory.
3. Is the “Longevity Dose” the same as the HIV dose? Answer: [Confidence: Medium] Likely Lower. The HED calculated from the mouse study (~57 mg q2d) is drastically lower than the standard HIV maintenance dose (600 mg/day). This suggests the senomorphic effect requires much less drug than viral entry inhibition.
4. Will this work if I just exercise? Answer: [Confidence: High] Exercise is likely synergistic. The paper implies that Maraviroc restores the capacity for regeneration. In a sedentary individual, this might maintain mass, but combined with resistance training (the signal for growth), the effects would likely be amplified.
5. Is Maraviroc a Senolytic (does it kill cells)? Answer: [Confidence: High] No, it is a Senomorphic. It blocks the signal (SASP) sent by senescent cells rather than inducing apoptosis (cell death). This is safer than senolytics (less toxicity) but requires continuous dosing to maintain the effect.
6. What are the liver risks? Answer: [Confidence: High] Real but Manageable. Hepatotoxicity is a known side effect. The label carries a warning. In a longevity context (elective use), baseline and monthly liver function tests (ALT/AST) would be non-negotiable.
7. Why use an HIV drug for aging? Answer: [Confidence: High] mechanism Repurposing. CCR5 is not just for HIV entry; it is a chemokine receptor involved in inflammation. Aging is essentially “inflammaging.” Blocking CCR5 dampens the specific inflammatory noise that prevents stem cells from repairing tissue.
8. Are there natural CCR5 antagonists? Answer: [Confidence: Low] Speculative. Some compounds like EGCG (Green Tea) and certain flavonoids have shown weak CCR5 modulation in silico or in vitro, but they lack the potency and specificity of Maraviroc.
9. Does this apply to women as well as men? Answer: [Confidence: Medium] Likely Yes. The mouse study used C57BL/6 mice (sex often specified as male in snippets, but sarcopenia mechanisms via SASP are generally conserved). However, immune responses can be sexually dimorphic, so human validation in females is needed.
10. What is the biggest “unknown” risk? Answer: [Confidence: Medium] Immune Compromise. CCR5 is part of the immune system. Long-term blockade in healthy (non-HIV) elderly people might impair the body’s ability to clear certain viral infections (e.g., West Nile Virus, influenza) or perform tumor surveillance, though HIV patients tolerate it well for years.
Reasoning Framework: Probabilistic & Bayesian
-
Priors: Sarcopenia is historically resistant to pharmacological intervention; exercise is the only proven treatment. “Senolytics” have shown promise in mice but failed in several human trials.
-
Update: This study provides strong mechanistic evidence (Atlas + In Vivo validation) that blocking CCR5 works. The effect size in mice (reversal of atrophy) is significant.
-
Confidence: We can be 80% confident in the mechanism (CCR5 drives muscle aging via SASP). We should be 40% confident in translation (that Maraviroc will safely reverse sarcopenia in humans) until Phase 2 trials confirm efficacy vs. side effects.
-
Alternative Hypothesis: The benefits might be due to systemic anti-inflammatory effects rather than specific “muscle stem cell” rejuvenation, meaning other broad anti-inflammatories could work similarly.
Sources: