Yes, interesting nuance. Well, you started the rabbit hole…
From the paper, other pathways remain unexplored, but inflammation rationalized to explain the difference in old aged vs young:
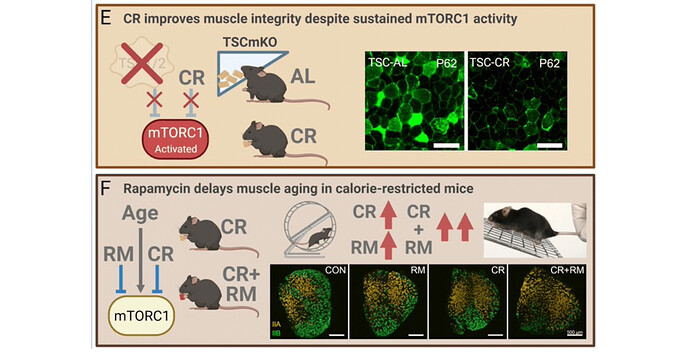
“Although UPP is one of the key pathways that degrade proteins, other pathways such as calpain, apoptosis, and autophagy-lysosome machinery also involve in cellular protein degradation. The role of other muscle degradation systems in the CR-induced preservation of muscle mass of older animals needs further studies. The underlying mechanism to explain the responses in mTOR signaling and UPP with CR and age is likely related to the cellular environment. The inflammatory
profile in skeletal muscles increases with aging. Thus, our finding of the concurrent upregulation of both anabolic and catabolic signaling pathways in aging muscles is likely a response to the inflammatory environment present in skeletal muscles of older animals. Because CR decreases inflammation, its impact is significant in the older animals compared to younger animals. Nevertheless, CR has potential adverse effects for certain biological systems and animals with specific genetic backgrounds (Contreras et al. 2018). For example, healthy individuals with long-term CR had lower leukocyte count which may compromise the defence function of their immune system compared to age-matched individuals who maintained a Western diet”
Why didn’t they measure INFLAMMATION markers in their mice??
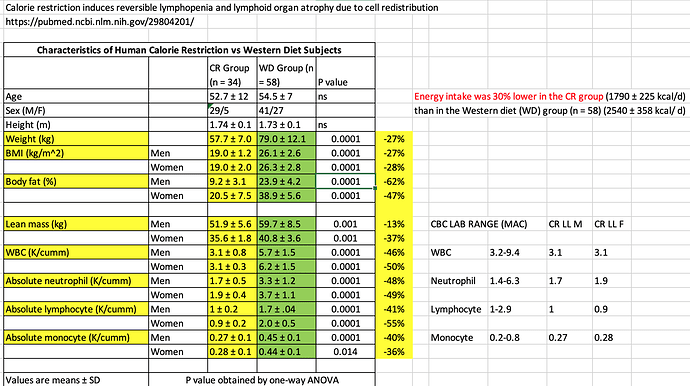
From the Contreras paper, I extracted the human CR metrics:
The immune metrics for the CR group are hovering at the LL values using the lab ranges where I am. So they aren’t significantly BELOW.
“From these data, we conclude that CR drives a reduction in total leukocyte cellularity, either by sequestration in tissues or a contraction/elimination during periods of reduced calories”
We know from the cancer/Sirolimus/GFJ paper (high dosing, cancer patients), Lymphopenia was a major adverse event, but this cohort of CR humans, those immune markers aren’t below lower limit.
Back to the CR mice:
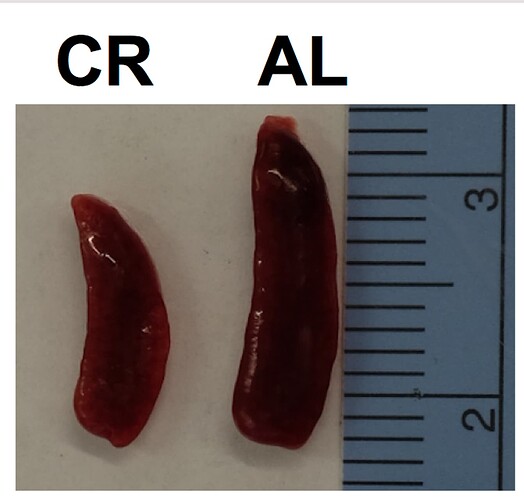
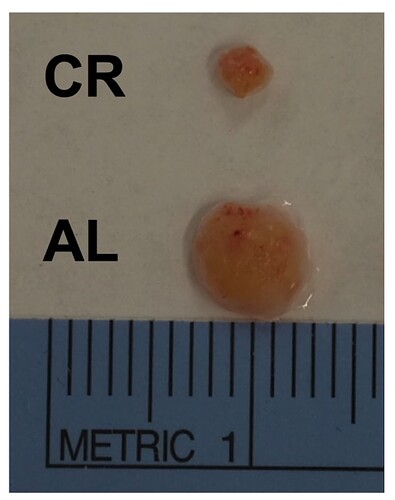
“In middle-aged 10- month-old mice, we found that during periods of caloric restriction, the absolute number of lymphocytes within the blood, inguinal and brachial lymph nodes, and spleen is reduced in comparison to AL fed counterparts. By contrast, the bone marrow of CR animals appeared to be a site of influx, or a safe haven for B, NK, and T cells, the numbers of which were at least intact, if not elevated in bone marrow. An obvious question of interest in this model relates to the functional consequences of CR-induced lymphopenia on immune defense. We and others have shown that systemic sepsis (Sun et al. 2001), epithelial and cutaneous infections all produce substantially higher mortality in old mice on lifelong CR. Less is known about vulnerability of mid-life animals in the course of CR induction, or for that matter, animals that are not held within specific pathogen free environments”
And in case you’re wondering about changes in spleen and thymus size in CR mice:
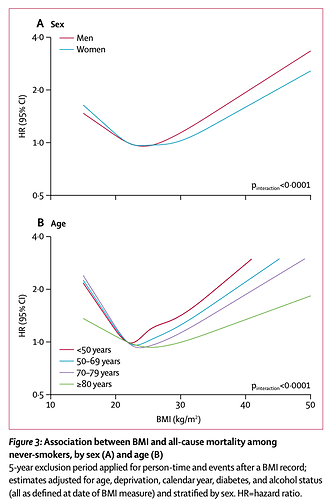
Some reference all-cause mortality (THE human metric to talk about in any longevity studies) and association with BMI:
https://www.thelancet.com/journals/landia/article/PIIS2213-8587(18)30288-2/fulltext
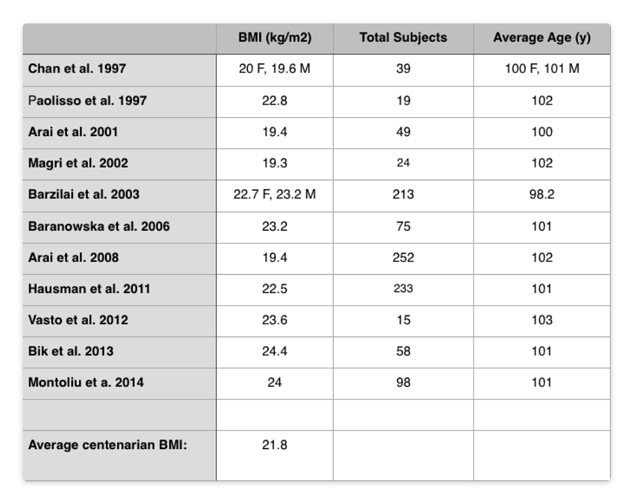
And for centenarians (average BM: 21.8)
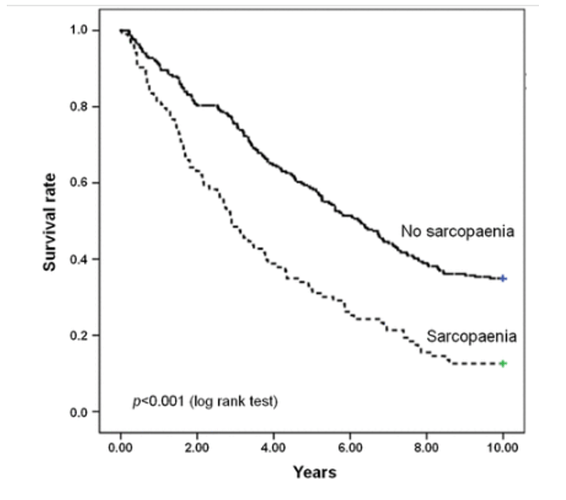
And how critically important is muscle and sarcopenia avoidance for longevity:
“Conclusions: Our findings show that physical function impairment, but not multimorbidity, is predictive of mortality in older community-dwellers with sarcopaenia. Hence, in sarcopaenic older persons, interventions against functional decline may be more effective at preventing or postponing negative health outcomes than those targeting multimorbidity.”
"Shockingly, the ill-effects of sarcopenia were virtually independent of whether or not subjects also had 2 or more conditions/diseases typical of old age, including: “obesity, coronary heart disease, cerebrovascular disease, congestive heart failure, peripheral artery disease, hypertension, lung disease (chronic obstructive pulmonary disease, emphysema or asthma), osteoarthritis, diabetes, dementia (Alzheimer’s disease and other forms of dementia), Parkinson’s disease, renal failure and cancer (non-melanoma skin cancer excluded).”
In other words, have sarcopenia, die early, regardless of what other ailments you do or do not have.