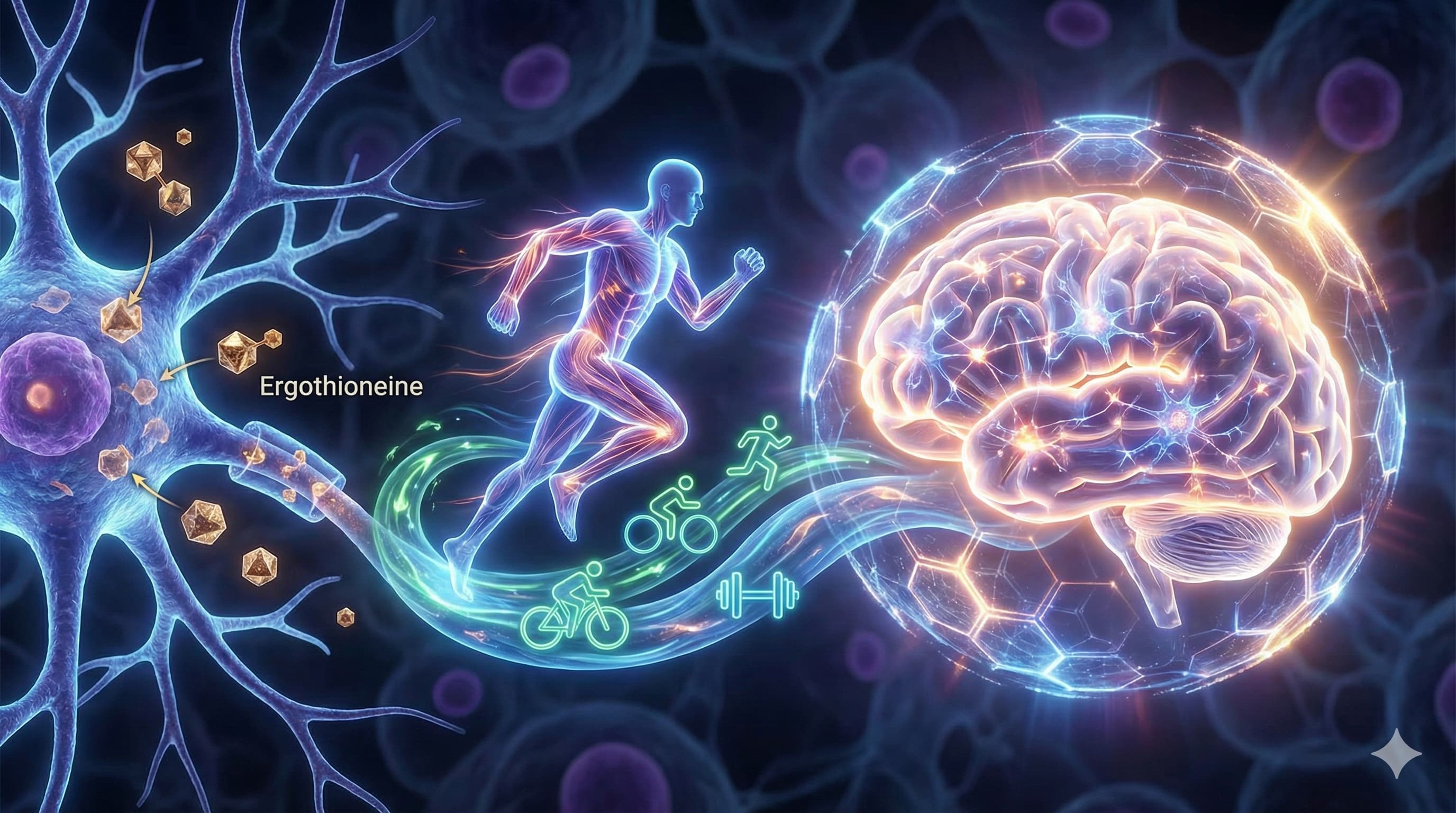
Cognitive decline remains the “Achilles’ heel” of human aging, frequently manifesting as neuroinflammation and mitochondrial decay decades before clinical symptoms arise. While pharmacological attempts to “clear” the brain of pathology have seen only marginal success, a new review from the NUS Academy for Healthy Longevity (Singapore), published in Ageing Research Reviews, proposes a potent non-pharmacological “one-two punch”: the combination of the rare dietary amino acid ergothioneine (ET) and structured physical exercise.
The “Big Idea” centers on biological complementarity. Ergothioneine is a unique sulfur-containing compound that humans cannot synthesize but must acquire from specific foods like mushrooms. It utilizes a dedicated transporter, OCTN1, to cross the blood-brain barrier and accumulate in high-stress tissues, where it acts as a “thione-based” antioxidant resistant to auto-oxidation. While exercise drives neurogenesis and improves cerebral blood flow, ET provides the cytoprotective “shield” required to protect those new neurons from oxidative stress. Critically, preliminary animal data suggests ET may even enhance exercise performance—extending time to exhaustion by 40%—without blunting the beneficial adaptive signaling (mitohormesis) that typically plagues traditional antioxidants like Vitamins C and E.
Paywalled Research Paper: Ergothioneine and exercise: A match made in (cognitive) heaven?
Impact Evaluation: The impact score of this journal is 12.4 (JIF 2024), evaluated against a typical high-end range of 0–60+ for top general science (e.g., Nature or The Lancet); therefore, this is a High impact journal within the specialized fields of gerontology and cell biology.
Part 2: The Biohacker Analysis
Study Design Specifications
- Type: Literature Review and Theoretical Conceptual Model (Synthesis of in vivo and clinical data).
- Subjects (Preclinical Data referenced): Rodents (Mice/Rats).
-
Lifespan Data: * Mice: Life-long administration of 4–5 mg/kg/day ET extended lifespan and attenuated cerebral decline in aging male mice.
- Note: Human lifespan data is currently observational/correlative.
Mechanistic Deep Dive
- Mitochondrial Dynamics: ET directly activates the MPST (3-mercaptopyruvate sulfurtransferase) enzyme, enhancing mitochondrial oxidative phosphorylation in muscle.
- Vascular Health: Aerobic exercise improves cerebral perfusion and functional connectivity, while ET protects brain endothelial cells from lipid-induced mitochondrial damage.
- Neurogenesis & BDNF: Exercise increases BDNF and IGF-1 expression; ET supplements this by dampening the neuroinflammatory environment (IL-1β, TNF-α) that typically inhibits new neuron survival.
Novelty
The paper identifies a “performance-preservation” paradox: most antioxidants (Vitamins C/E) inhibit mitochondrial biogenesis (PGC-1α). ET is novel because it reduces oxidative stress without impairing adaptive exercise responses, potentially acting as a “smart” antioxidant.
Critical Limitations
- Translational Gap: There are currently zero human trials testing the combined effects of ET and exercise on cognition.
- Dosing Uncertainty: Human trials have been limited to 10–25 mg/day; it is unknown if higher doses provide superior benefits.
- Sample Size: Human pilot studies (e.g., Yau et al., 2024) are small (N=19), limiting statistical power.
Part 3: Actionable Intelligence
The Translational Protocol (Rigorous Extrapolation)
-
Human Equivalent Dose (HED): * Calculation: Based on the mouse dose of 1 mg/day (typical human mirror).
- BSA Normalization: For a 70 kg human, the HED extrapolated from mouse longevity studies (4∼5 mg/kg) is approximately 23∼28 mg/day.
- Pharmacokinetics (PK/PD): ET has a remarkably long half-life in humans (approx. 30 days due to renal reabsorption via OCTN1).
-
Safety & Toxicity Check: * NOAEL: Generally recognized as safe (GRAS) by the FDA and EFSA.
- Toxicity: No signs of toxicity observed in 12-month human trials at 25 mg 3x/week.
- LD50: Data is scarce but exceeds 2000 mg/kg in acute animal models.