A new review paper on the NRF2 Pathway.
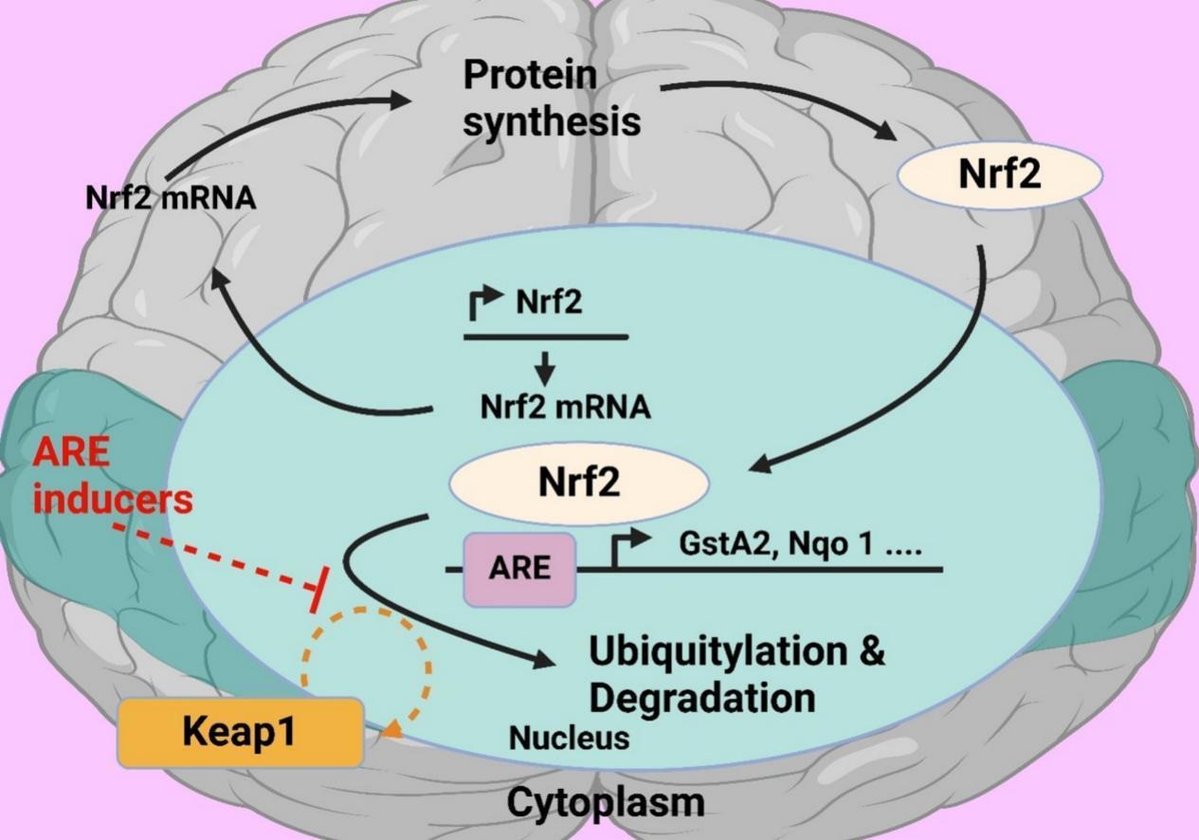
For a long time Nrf2 transcription factor has been attracting attention of researchers investigating phenomenon of aging. Numerous studies have investigated effects of Nrf2 on aging and cell senescence. Nrf2 is often considered as a key player in aging processes, however this needs to be proven. It should be noted that most studies were carried out on invertebrate model organisms, such as nematodes and fruit flies, but not on mammals. This paper briefly presents main mechanisms of mammalian aging and role of inflammation and oxidative stress in this process. The mechanisms of Nrf2 activity regulation, its involvement in aging and development of the senescence-associated secretory phenotype (SASP) are also discussed. Main part of this review is devoted to critical analysis of available experimental data on the role of Nrf2 in mammalian aging.
CONCLUSION
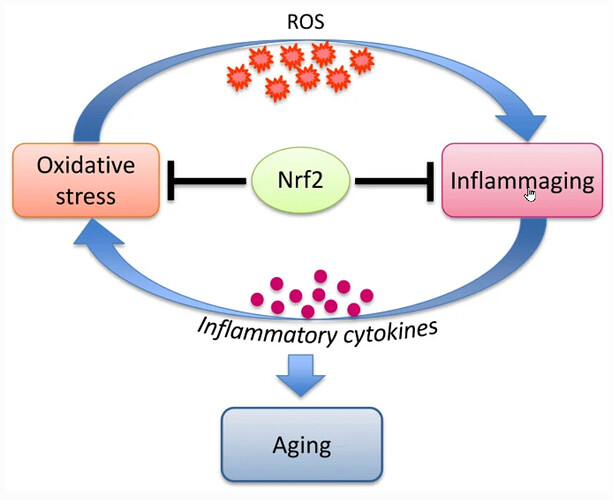
Aging in organisms is accompanied by (i) accumulation of oxidative damage and (ii) development of chronic inflammation, “inflammaging”. Activation of the transcription factor Nrf2 may affect both of these factors, slowing down development of the senile changes. An indirect confirmation of this assumption is the fact that the long-lived animals, such as naked mole rat, have an increased level of Nrf2 activation. An important area of research should aim to obtain independent experimental data on the age-related dynamics of Nrf2 activity changes in animals and humans, since there is no data of this kind.
It is tempting to speculate that in order to successfully fight aging, humans must learn how to properly activate Nrf2, like naked mole rats do. However, we should consider that the long-lived organisms have evolved to adapt to high Nrf2 activity and fine-tuned complex systems of interactions of the signaling and metabolic pathways. Therefore, simple pharmacological activation of Nrf2 to prolong life does not seem to be the most promising approach, moreover, it could increase the risks of serious side effects. There is no reliable data in the literature that unequivocally prove that Nrf2 activation actually leads to increase of the lifespan of mammals.
An open access paper:
Hoping a little NRF2 activation is a good thing I use Moringa powder (which tastes terrible) in my frequent morning smoothie with a lot of frozen berries to mask the taste of the moringa.