Nice. The empa study is in obesity induced rats. Would be interesting to see general effects in non morbid rats. Of course they’d have to select different criteria, as there would be no NAFLD etc. The dapa study in humans is great, can’t wait for more.
05/03/2021 - 174ng/ml
11/25/2024 - 200ng/ml (~2 months of Empagliflozin 25mg/day)
01/02/2025 - 206ng/ml
Of course, many changes between 2021 and 2024, but I’m not aware of any other changes I’ve made that scream impact on IGF-1. If anything, 3 years of aging should have reduced it slightly. I’m 32 for reference.
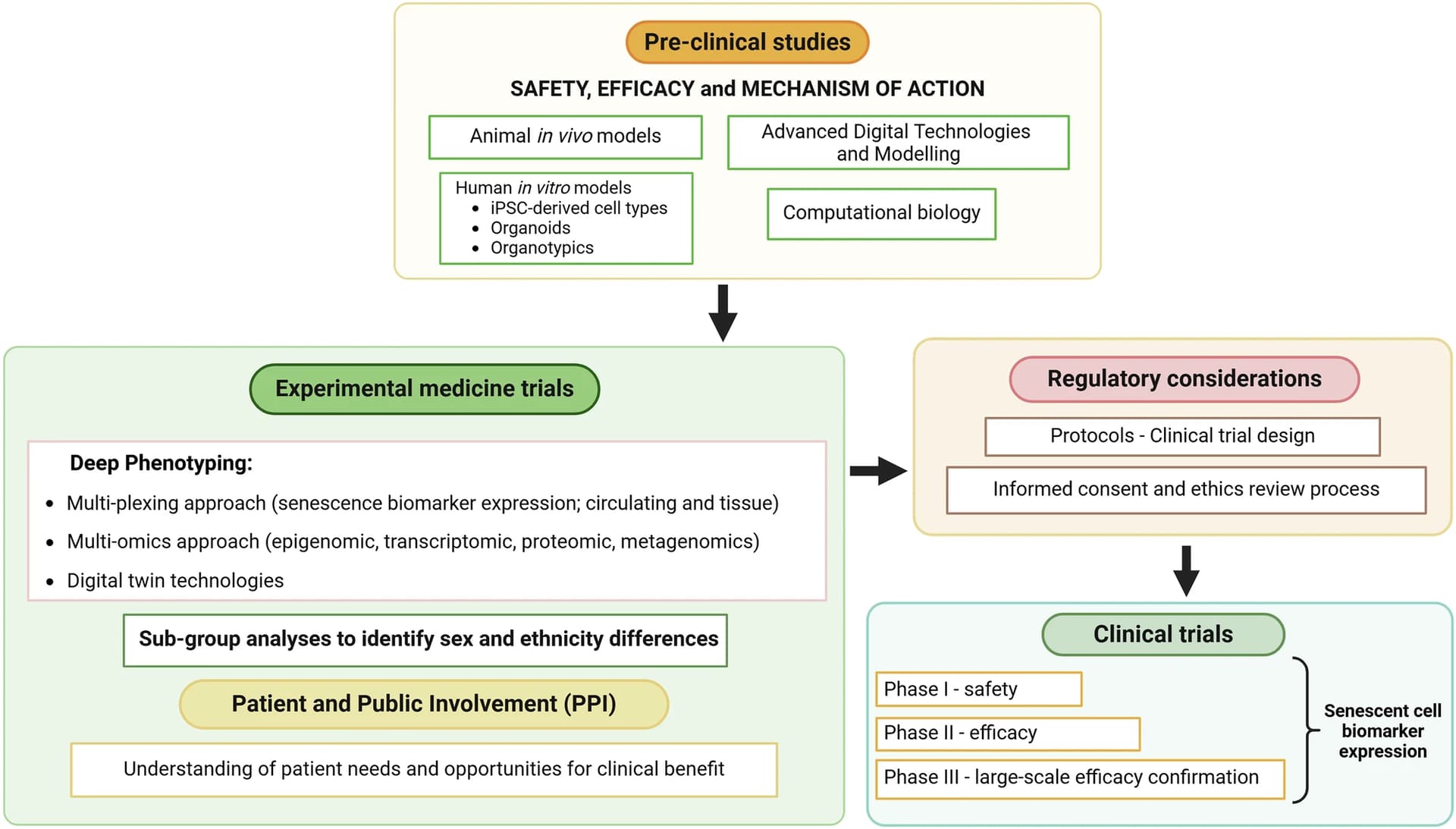
SGLT2 inhibitors as a novel senotherapeutic approach 2025
King’s College London + Imperial College + Queen Mary University
Cellular senescence is the permanent cessation of cell proliferation and growth. Senescent cells accumulating in tissues and organs with aging contribute to many chronic diseases, mainly through the secretion of a pro-inflammatory senescence-associated secretory phenotype (SASP). Senotherapeutic (senolytic or senomorphic) strategies targeting senescent cells or/and their SASP are being developed to prolong healthy lifespan and treat age-related pathologies. Sodium-glucose co-transporter 2 (SGLT2) inhibitors are a new class of anti-diabetic drugs that promote the renal excretion of glucose, resulting in lower blood glucose levels. Beyond their glucose-lowering effects, SGLT2 inhibitors have demonstrated protective effects against cardiovascular and renal events. Moreover, SGLT2 inhibitors have recently been associated with the inhibition of cell senescence, making them a promising therapeutic approach for targeting senescence and aging. This review examines the latest research on the senotherapeutic potential of SGLT2 inhibitors.
In conclusion, the present findings indicate the clinical potential of SGLT2 inhibition in preventing or delaying age-related diseases through a plethora of mechanisms. Thus, further studies are required to elucidate the efficacy and safety of SGLT2 inhibitors as a senotherapeutic for age-related pathologies.
Most of the sglt2i are in slow release tabs that shouldn’t be split. Is dapa different?
A doctor told me to split them. This is a list of inactive ingredients on the first search result hit. None of them indicate enteric coating or slow release. Two indicate the opposite.
Microcrystalline cellulose: A filler and binder, often used in immediate-release and controlled-release formulations but not specific to either.
Anhydrous lactose: A filler or diluent, typically in immediate-release tablets.
Crospovidone: A disintegrant that promotes rapid tablet breakdown for immediate release.
Silicon dioxide: A glidant or anti-caking agent, not related to release properties.
Magnesium stearate: A lubricant to aid tablet manufacturing, not specific to release type.
Grok confirms:
Dapagliflozin, sold under brand names like Farxiga (US) and Forxiga (EU), is a sodium-glucose cotransporter 2 (SGLT2) inhibitor primarily used for type 2 diabetes, heart failure, and chronic kidney disease. Based on available information, dapagliflozin is typically formulated as an immediate-release tablet, not as an enteric-coated or slow-release (sustained-release) formulation.Key Points:Formulation: Dapagliflozin tablets are film-coated, but the coating is not described as enteric or designed for delayed or controlled release. The film coating is primarily for stability, ease of swallowing, or aesthetic purposes, not for modifying release kinetics. Pharmacokinetic data indicate that dapagliflozin reaches maximum plasma concentration (Cmax) within 1-2 hours after oral administration in a fasting state, consistent with immediate-release formulations.No Enteric Coating: There is no evidence from standard references, including the drug’s package insert or pharmacological profiles, suggesting that dapagliflozin requires or commonly uses an enteric coating. Enteric coatings are typically used for drugs that are acid-labile, irritate the stomach, or target intestinal release (e.g., proton pump inhibitors or aspirin). Dapagliflozin does not have these characteristics, as it is stable in gastric pH and absorbed primarily in the small intestine after rapid dissolution.No Slow-Release Formulation: Dapagliflozin is not marketed as a slow-release or extended-release product. Its mechanism of action relies on inhibiting SGLT2 in the kidneys to promote glucose excretion, which does not necessitate prolonged release. The drug’s half-life (approximately 12.9 hours) supports once-daily dosing without the need for modified-release technology.Clinical Use: The immediate-release nature of dapagliflozin aligns with its therapeutic goal of achieving rapid and consistent glucose-lowering effects. Modified-release formulations are more common for drugs requiring reduced dosing frequency or minimized peak-trough fluctuations, which is not a primary concern for dapagliflozin.Supporting Evidence:The FDA-approved prescribing information for Farxiga describes dapagliflozin as a film-coated tablet available in 5 mg and 10 mg strengths, with no mention of enteric or controlled-release properties.Pharmacokinetic studies show that dapagliflozin’s absorption is rapid, with urinary glucose excretion effects observed soon after administration, further supporting an immediate-release profile.Unlike medications such as enteric-coated aspirin or extended-release metformin (e.g., Glucophage XR), dapagliflozin’s formulation does not include polymers like hydroxypropyl methylcellulose (HPMC) or methacrylic acid copolymers (e.g., Eudragit) commonly used for enteric or slow-release coatings.Conclusion:Dapagliflozin does not have to be and is not usually enteric-coated or slow-release. It is formulated as an immediate-release, film-coated tablet for rapid absorption and onset of action. If you have a specific brand or formulation in mind, please provide additional details, and I can investigate further. Always consult your healthcare provider for guidance on your specific medication.
I don’t believe that is accurate, I think most of them can be split. The companies like to promote this idea so you won’t save money and will buy the lower dose/ higher price tablets, but I think they likely work just as well split. See this thread: Which pills can be split and in what dose and which cannot be split
I have split dapa and monitored with a cgm. I did not see any issue.
and I have split empagliflozin and monitored with CGM and all works as expected (greatly reduced blood glucose spikes).
Is it possible for me to use this if I am on tirzepatide and berberine?
Another question: If these drugs cause you to pee out the sugar, would this mean you don’t feel the energy of the sugar that you’ve eaten? I ask this in the context of exercise, I consume carbohydrates before exercise. Would this blunt that energy?
Health data is very powerful. I had a similar experience. I unfortunately have sleep apnea, and shortly after getting my CPAP machine I drank some alcohol and that night had alarmingly high AHI the next morning. I literally have not touched alcohol since then, it has been years.
How are you all procuring SGLTi unless you have a formal indication? Indian pharmacy?
I’m sure my doc would write an rx if I wanted him to, just as he did for metformin that I didn’t ‘need’, but regardless, I do get them sent from India for cost reasons. There are many sources available, and I think that is what most, if not all, of us do.
Most of us who have been using SGLTi’s have been getting them from India, but now companies are prescribing them in the US, for example Healthspan: Doctor-Prescribed SGLT-2 Inhibitor for Longevity and Healthspan
I think AgelessRX is also doing it, or will be soon. But generally doctors are not prescribing these drugs to healthy people yet.
The flozins are generally too expensive in the USA for most to consider. India provides a cost-effective quality solution.
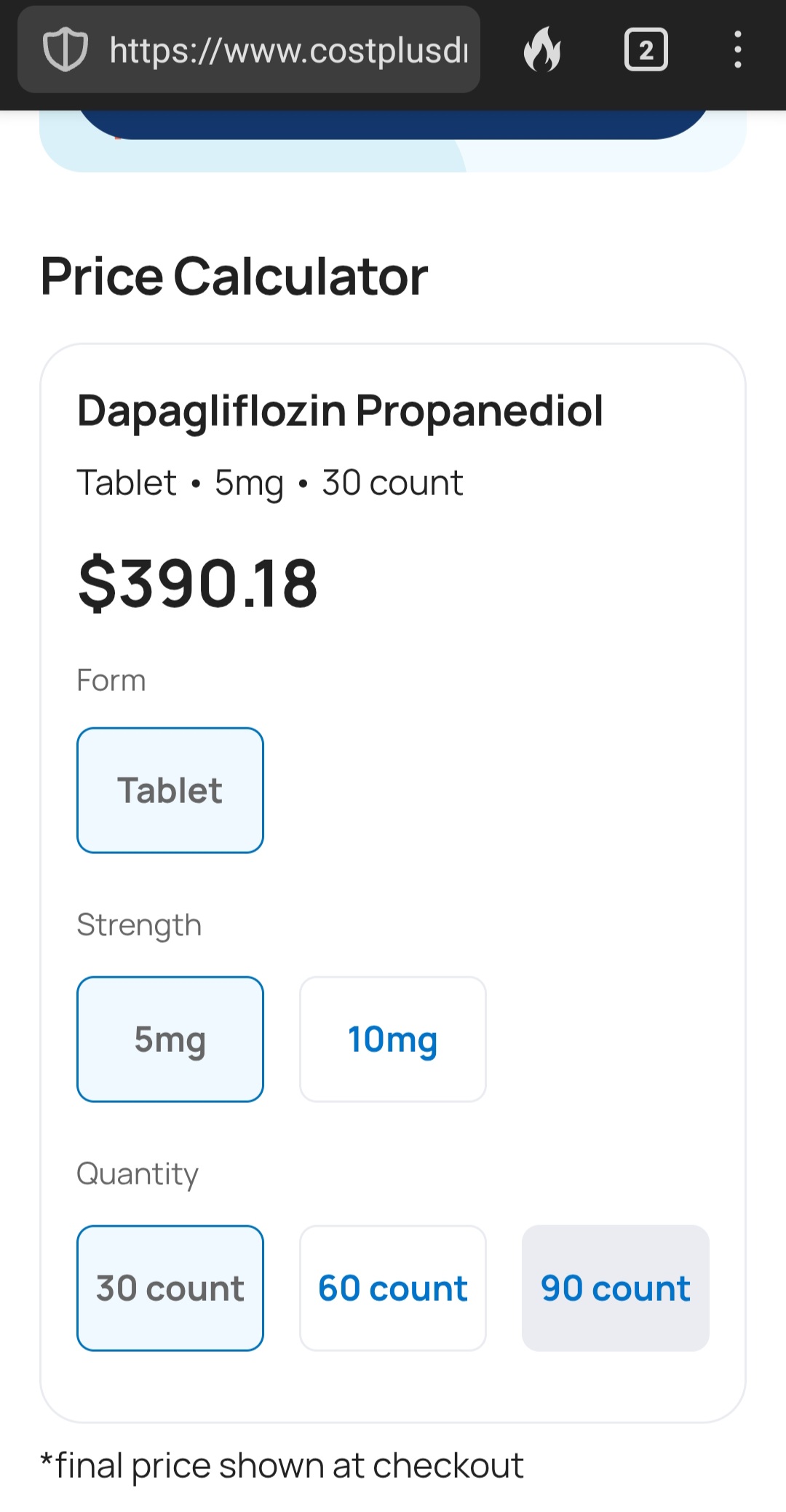
Yeah, I looked it up at Mark Cuban’s Costplus (which is most economical of all pharmacies) and its over 10 bucks a pill
India is around $60 for a month’s supply, or about $2 per pill.
Aren’t these going generic soon?
They are starting to… and there seems to be at least one that may be relatively inexpensive:
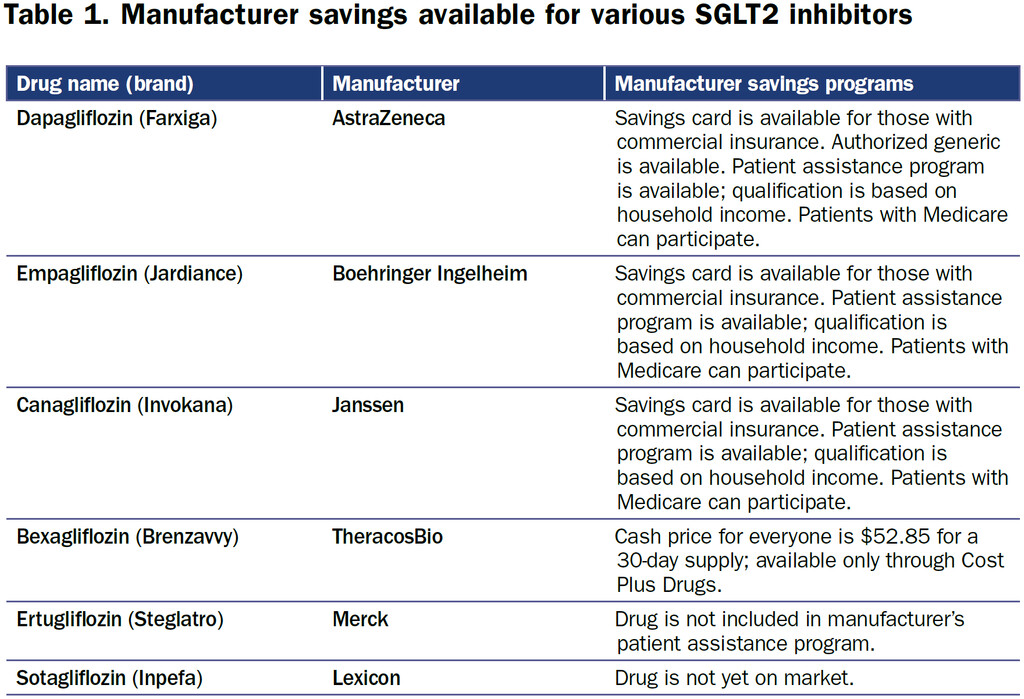
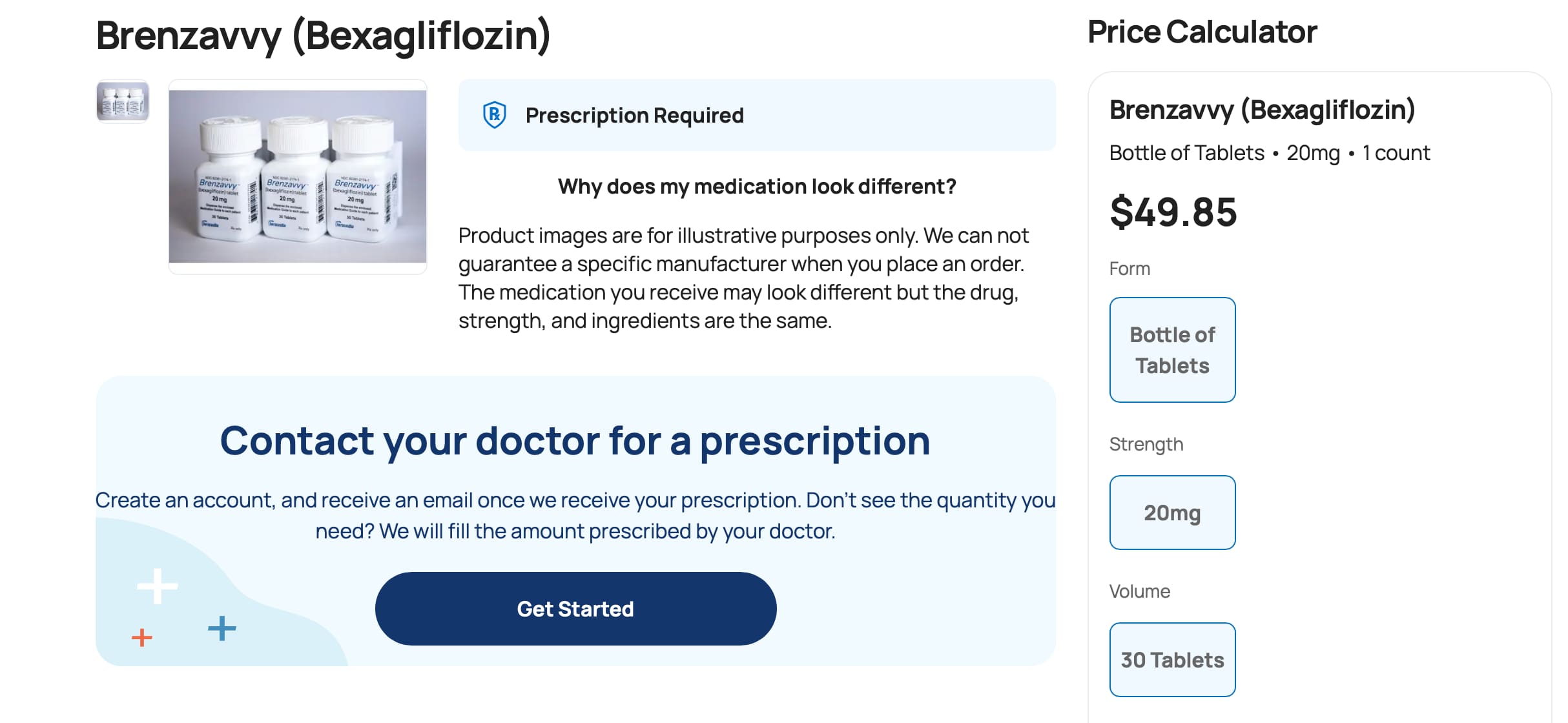
The SGLT2i bexagliflozin was approved in January 2023 as adjunctive therapy to lower blood glucose in patients with type 2 diabetes. Patients with an estimated glomerular filtration rate less than 30 are excluded on the label (4). Bexagliflozin (brand name Brenzavvy) was not expected to have much success as a late entrant into the field, especially since established SGLT2i, including dapagliflozin (Farxiga), canagliflozin (Invokana), and empagliflozin (Jardiance), have additional cardiovascular and kidney-labeled indications. However, in a novel marketing approach, bexagliflozin is available exclusively through Cost Plus Drugs at a cash price of approximately $50 per month (5). There are no required patient assistance programs or other qualifying steps to access the medication (Table 1). Prescribing physicians should exercise their best clinical judgment to determine if the evidence for a class effect of the SGLT2i is enough to warrant off-label use of bexagliflozin to provide benefit among patients with cardiovascular and kidney diseases.
AstraZeneca’s Farxiga (dapagliflozin) does not go off patent until 2025, and in 2021, AstraZeneca successfully defended its patent against a challenge from a manufacturer of generic medications (6). However, generic dapagliflozin is now available in the United States. In a marketing strategy familiar to Costco shoppers, who purchase brand name products sold under the Kirkland label at a significant discount, AstraZeneca has licensed Prasco to sell dapagliflozin as an authorized generic. The medication is manufactured in the same facility as the branded product and is identical other than the packaging (7). There is the potential for savings to be passed on to the consumer, although the savings may also be captured by pharmacy benefit managers and the pharmacies.
Source: Newer Options for SGLT2 Inhibitors in the United States in: Kidney News Volume 16 Issue 4 (2024)
Related:
FDA Moves Generic Dapagliflozin a Step Closer to US Sales
Mitchel L. Zoler, PhD
February 23, 2022
However, the Zydus formulations of dapagliflozin will likely not soon appear on the US market because in October 2021 the company failed in its bid in US District Court to invalidate the patent that AstraZeneca holds on dapagliflozin through 2025 based on a claim of “obviousness.” The court dismissed the Zydus claims and upheld the validity of the patent.
https://www.medscape.com/viewarticle/969050
https://www.goodrx.com/classes/sglt2-inhibitors/sglt2-inhibitors-list
That’s very interesting.
Looks like it’s as good as dapagliflozin:
Efficacy and safety of bexagliflozin compared with dapagliflozin as an adjunct to metformin in Chinese patients with type 2 diabetes mellitus: A 24-week, randomized, double-blind, active-controlled, phase 3 trial
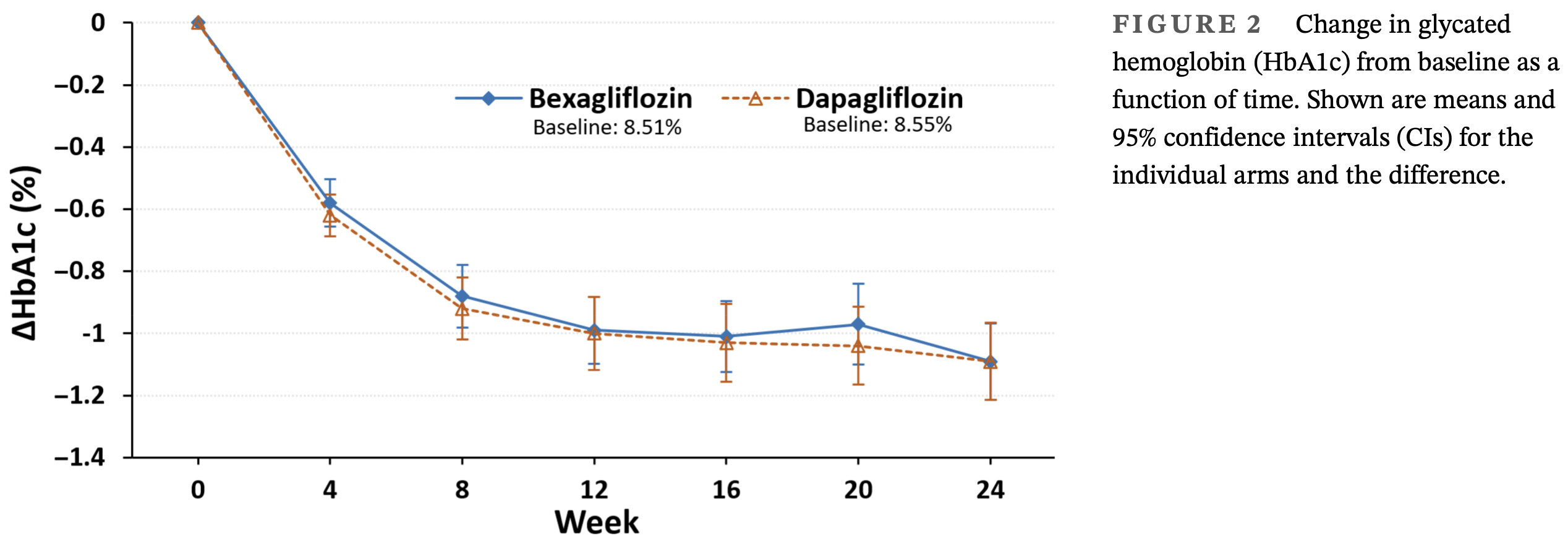
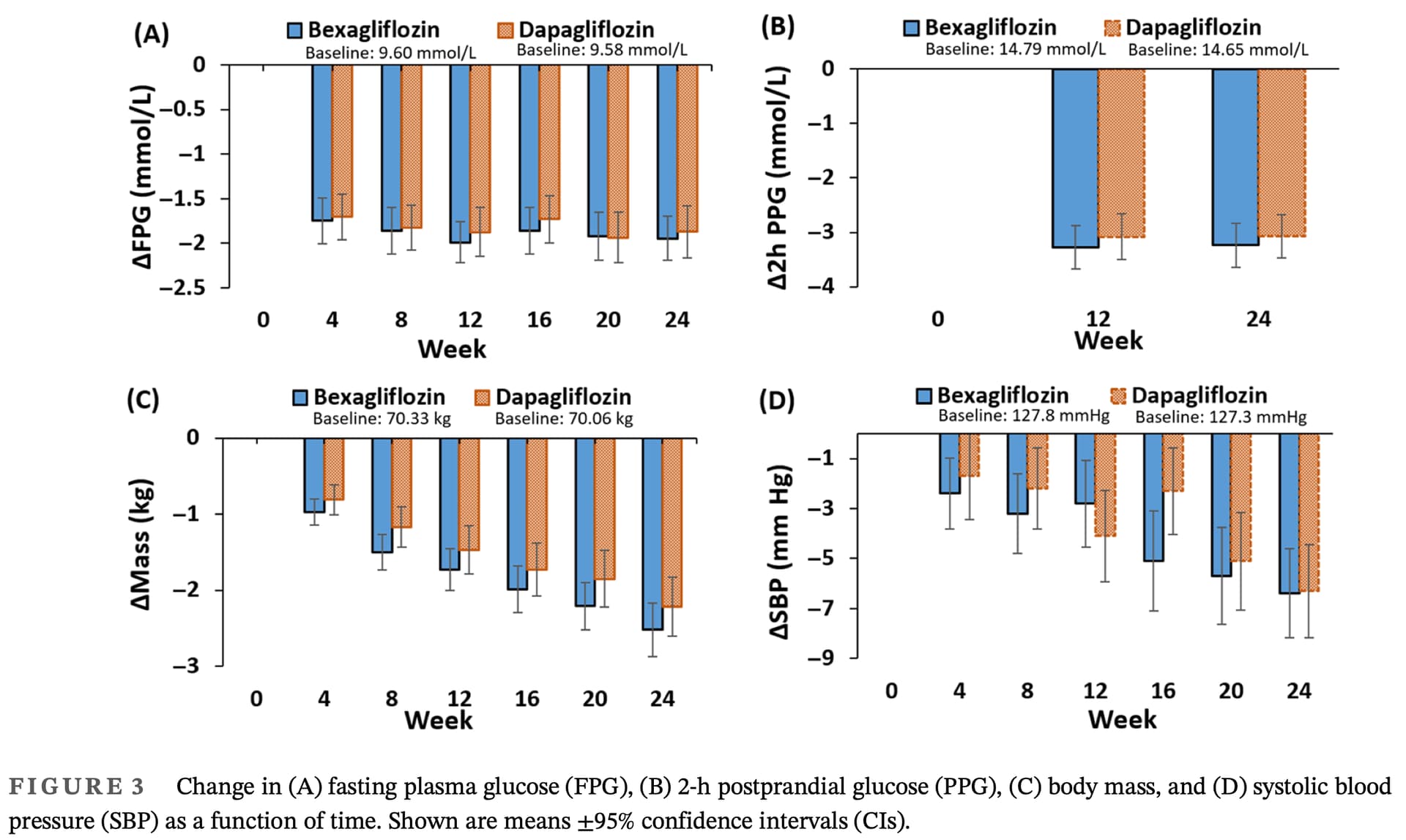
For the primary endpoint of change in HbA1c from baseline to week 24, the main objective to demon-
strate that bexagliflozin was noninferior to dapagliflozin
was achieved. The similar reductions in HbA1c observed
with bexagliflozin and dapagliflozin suggest that both
SGLT2 inhibitors are effective in glycemic control.