The flozins are generally too expensive in the USA for most to consider. India provides a cost-effective quality solution.
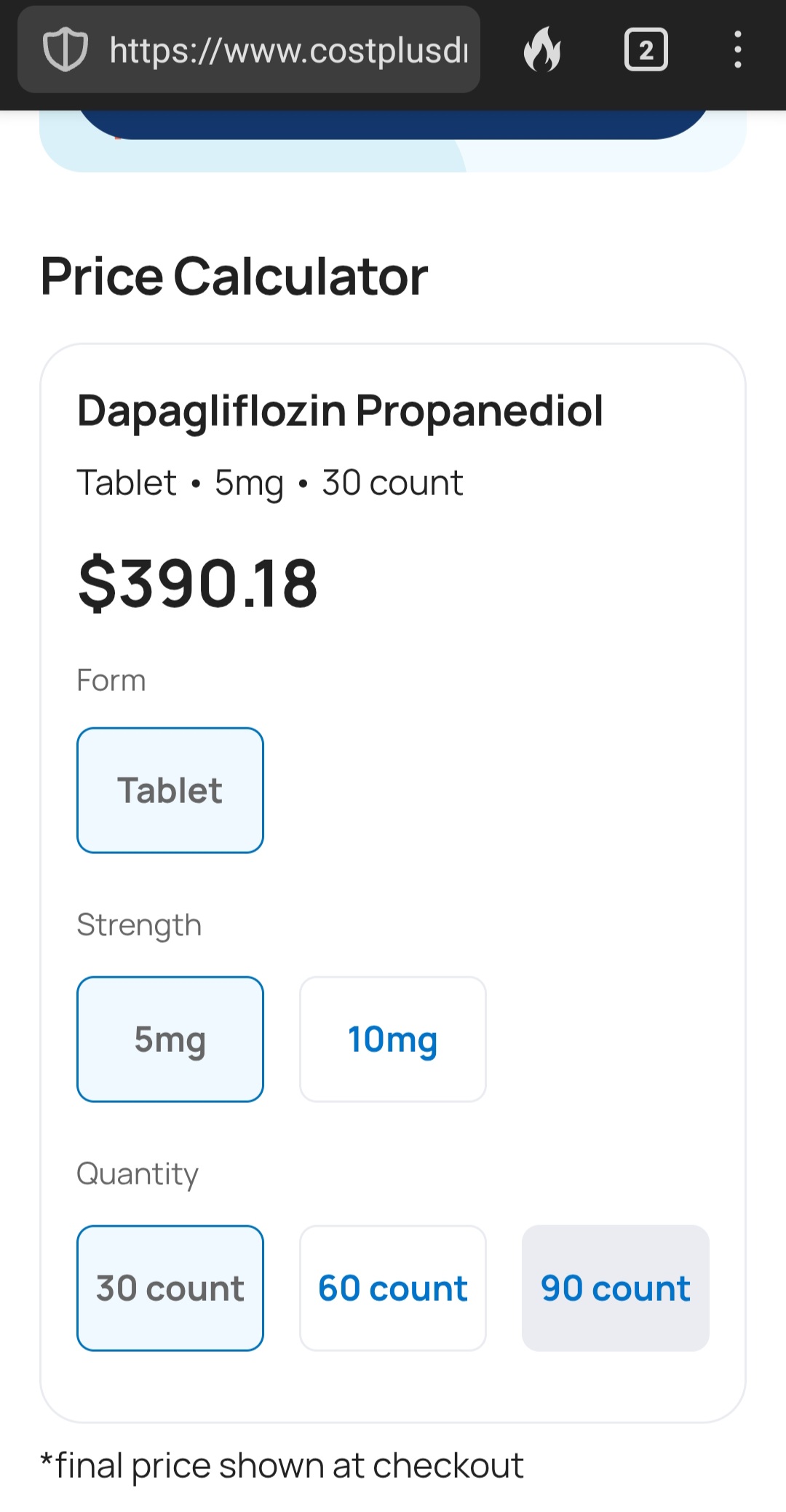
Yeah, I looked it up at Mark Cuban’s Costplus (which is most economical of all pharmacies) and its over 10 bucks a pill
India is around $60 for a month’s supply, or about $2 per pill.
Aren’t these going generic soon?
They are starting to… and there seems to be at least one that may be relatively inexpensive:
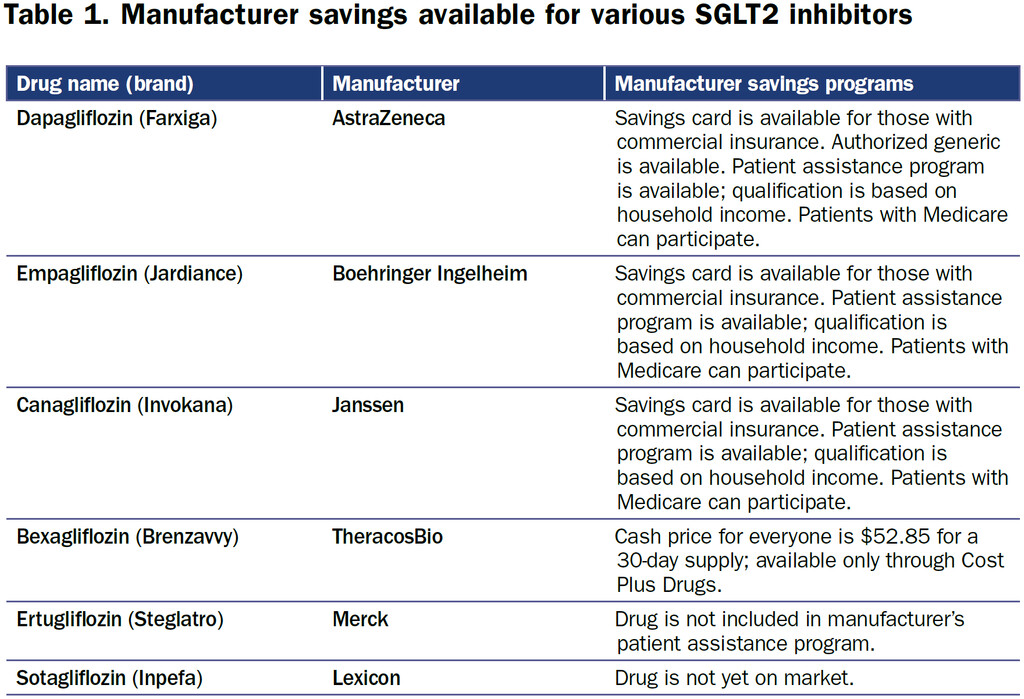
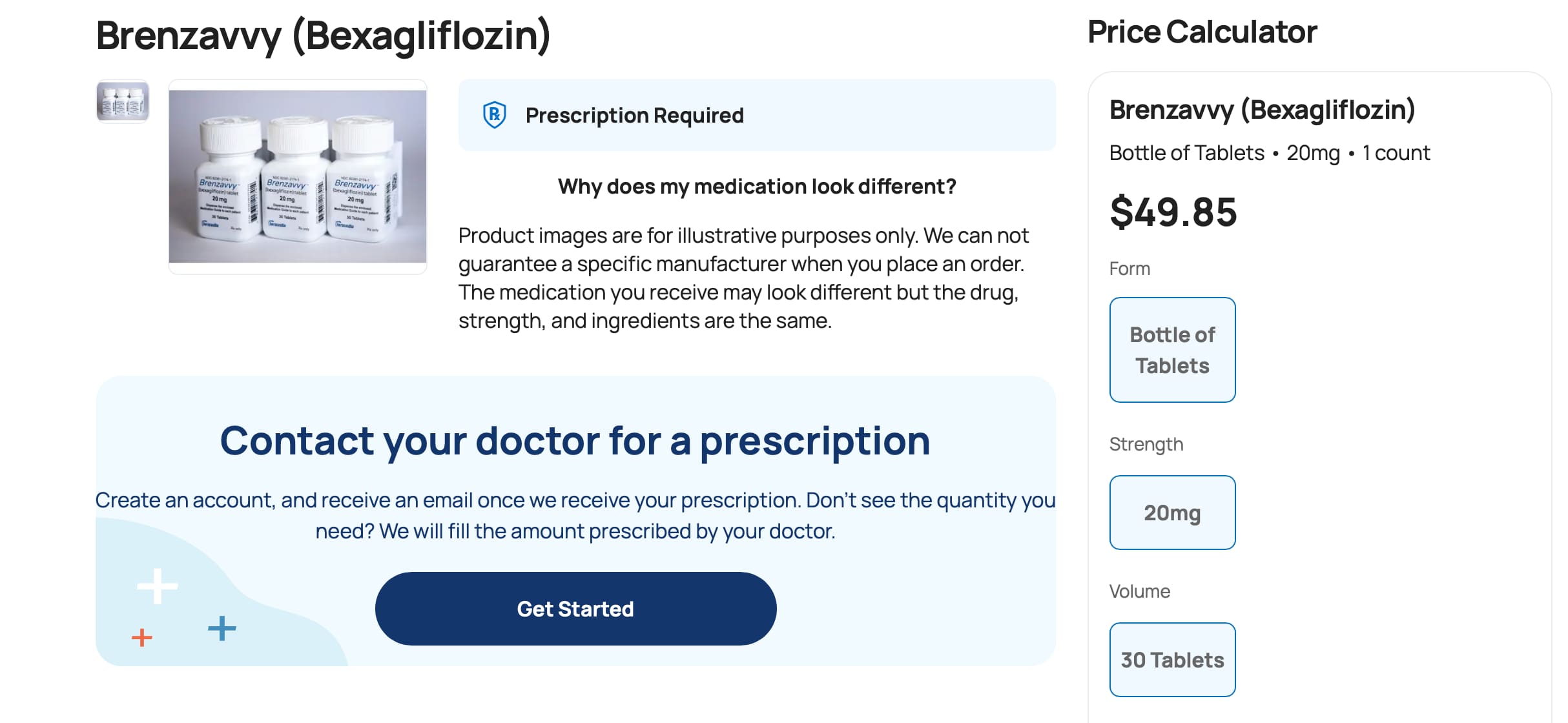
The SGLT2i bexagliflozin was approved in January 2023 as adjunctive therapy to lower blood glucose in patients with type 2 diabetes. Patients with an estimated glomerular filtration rate less than 30 are excluded on the label (4). Bexagliflozin (brand name Brenzavvy) was not expected to have much success as a late entrant into the field, especially since established SGLT2i, including dapagliflozin (Farxiga), canagliflozin (Invokana), and empagliflozin (Jardiance), have additional cardiovascular and kidney-labeled indications. However, in a novel marketing approach, bexagliflozin is available exclusively through Cost Plus Drugs at a cash price of approximately $50 per month (5). There are no required patient assistance programs or other qualifying steps to access the medication (Table 1). Prescribing physicians should exercise their best clinical judgment to determine if the evidence for a class effect of the SGLT2i is enough to warrant off-label use of bexagliflozin to provide benefit among patients with cardiovascular and kidney diseases.
AstraZeneca’s Farxiga (dapagliflozin) does not go off patent until 2025, and in 2021, AstraZeneca successfully defended its patent against a challenge from a manufacturer of generic medications (6). However, generic dapagliflozin is now available in the United States. In a marketing strategy familiar to Costco shoppers, who purchase brand name products sold under the Kirkland label at a significant discount, AstraZeneca has licensed Prasco to sell dapagliflozin as an authorized generic. The medication is manufactured in the same facility as the branded product and is identical other than the packaging (7). There is the potential for savings to be passed on to the consumer, although the savings may also be captured by pharmacy benefit managers and the pharmacies.
Source: Newer Options for SGLT2 Inhibitors in the United States in: Kidney News Volume 16 Issue 4 (2024)
Related:
FDA Moves Generic Dapagliflozin a Step Closer to US Sales
Mitchel L. Zoler, PhD
February 23, 2022
However, the Zydus formulations of dapagliflozin will likely not soon appear on the US market because in October 2021 the company failed in its bid in US District Court to invalidate the patent that AstraZeneca holds on dapagliflozin through 2025 based on a claim of “obviousness.” The court dismissed the Zydus claims and upheld the validity of the patent.
https://www.medscape.com/viewarticle/969050
https://www.goodrx.com/classes/sglt2-inhibitors/sglt2-inhibitors-list
That’s very interesting.
Looks like it’s as good as dapagliflozin:
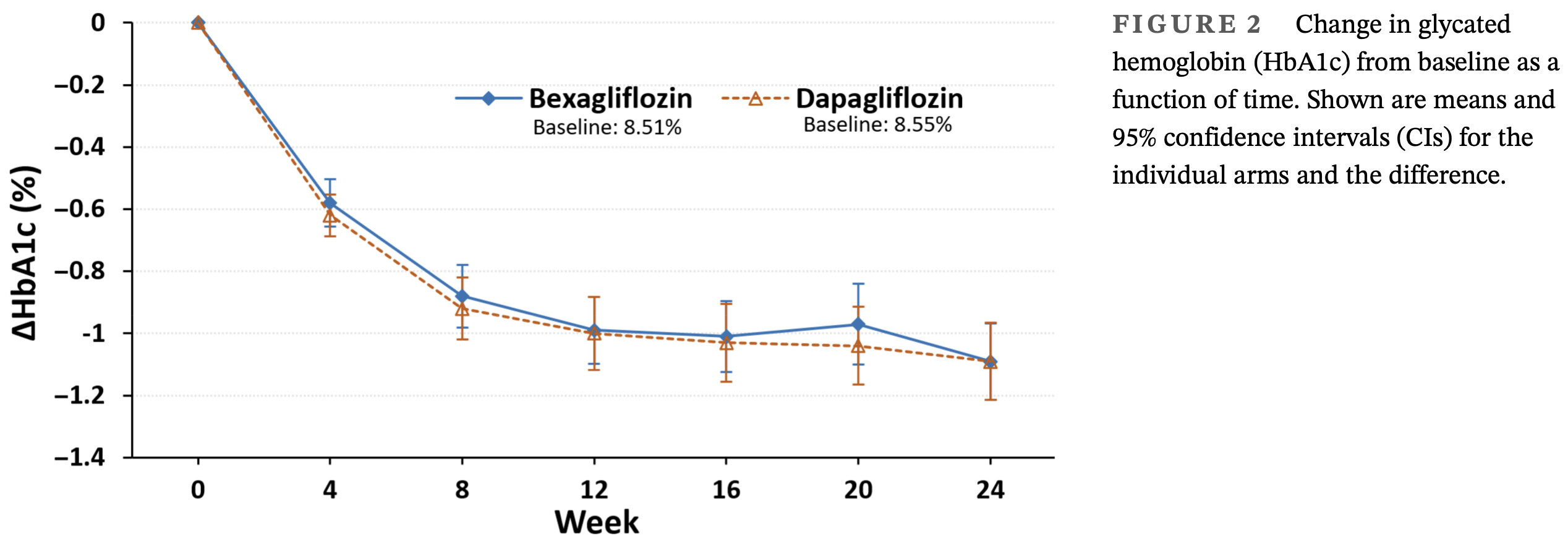
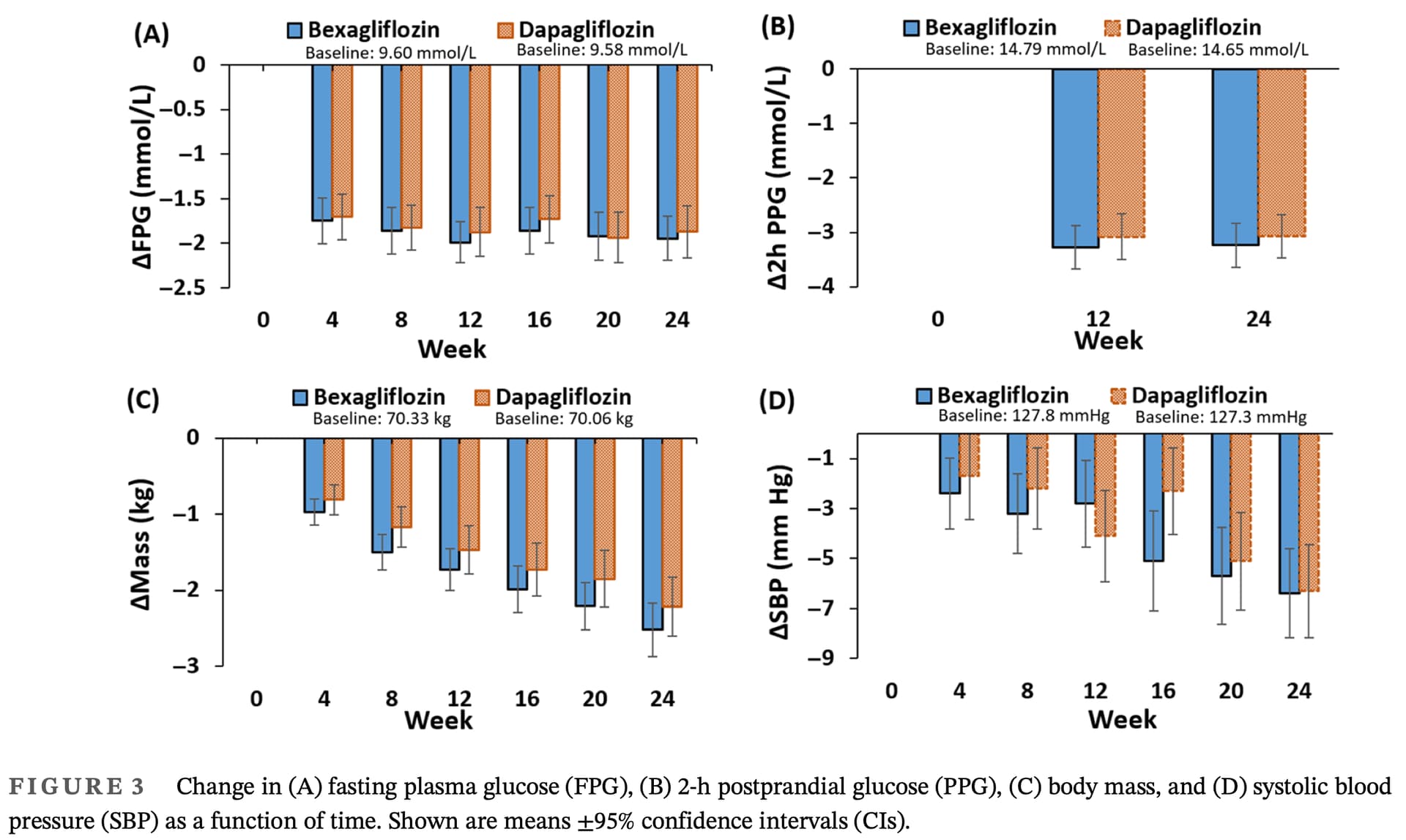
Efficacy and safety of bexagliflozin compared with dapagliflozin as an adjunct to metformin in Chinese patients with type 2 diabetes mellitus: A 24-week, randomized, double-blind, active-controlled, phase 3 trial
For the primary endpoint of change in HbA1c from baseline to week 24, the main objective to demon-
strate that bexagliflozin was noninferior to dapagliflozin
was achieved. The similar reductions in HbA1c observed
with bexagliflozin and dapagliflozin suggest that both
SGLT2 inhibitors are effective in glycemic control.
LOL, I did the same thing - immediately started scouring PubMed for info about bexagliflozin. I did find that it works in cats, which is always nice to know (for us, cat owners) as diabetes is very prevalent among cats. So it’s comforting to know that as you’re popping your SGLT2i, you might give some to your cat - the little guys are on the same journey as we are: rapamycin, sglt2i etc. I posted the relevant study in a cat thread although a bit OT as the thread is about rapamycin.
I’ve also seen the one on humans and cat. I found it was rather funny.
I asked Consensus about a non-diabetic taking an SGLT2 inhibitor for longevity and the response was below. Would be interested if anyone has thoughts about this response:
based on current evidence, I would not recommend that a healthy, non-diabetic person take empagliflozin (or any SGLT2 inhibitor) for general health or longevity. Here’s why:
- No Proven Benefit in Healthy People
- There are no clinical trials showing that empagliflozin improves health outcomes or extends lifespan in non-diabetics. Most studies focus on diabetics or those with heart failure or chronic kidney disease.
- Mechanism Doesn’t Offer Advantage Without Hyperglycemia
- Empagliflozin works by causing excess glucose to be excreted in urine. If your glucose levels are normal, there’s little to excrete—so the drug does almost nothing.
- Risks Without a Clear Benefit
-
Known side effects include:
- Genital and urinary tract infections
- Volume depletion and dehydration
- Electrolyte imbalances
- Rare risk of diabetic ketoacidosis (DKA), even in non-diabetics
(Simes & MacGregor, 2019)
SGLT2i offer kidney protection in CDK patients with and without diabetes.
I’d go back through this thread and look at the many studies shared, because the above statement is simply false. SGLT2 receptors are found in multiple organs/tissues, including the heart, blood vessels, brain, etc. Mechanisms for beneficial effects exist that have nothing to do with glucose excretion in the urine.
My thoughts are that this response is a very good example of why “Consensus” belongs in the circular file and subsequent wastebasket. Other posters have already indicated the laughable lack of evidence being incorporated from easily available studies. But with performance this bad, why would anyone ever use this, when there is no way to know when it’s being actively informationally destructive. It’s like going to the doctor whose job it is to cure you, but you never know when he might randomly shoot you in the head. I’d guess that the number of his patients would be zero.
@Virilius and @CronosTempi
Yes! I sent the information to my vet because, while my cats don’t have diabetes, they do have CKD, so I asked her if I can share my stash of dapagliflozin! (She approves of me sharing my rapa with them). Stay tuned !
Appreciate the responses. I think consensus is probably just overly conservative. This is what it said about some of those other studies mentioned:
Despite all these promising mechanisms, here’s the key:
• Clinical benefit has only been proven in people with specific risk factors—CKD, heart failure, or diabetes.
• In healthy individuals:
• You may not have enough physiological “stress” (e.g., high glomerular pressure, sodium retention, inflammation) for these mechanisms to meaningfully engage.
• There’s no safety data or long-term outcome studies.
• Side effects (e.g., dehydration, infections, rare DKA) are real and may outweigh any theoretical benefit.
⸻
Conclusion:
Yes—SGLT2 inhibitors work through multiple mechanisms that go far beyond glucose lowering. These explain their powerful benefits in people with heart failure or CKD, even without diabetes. But no studies show that these benefits translate to healthy people, so use in that context remains speculative and unadvised for now.
It’s perfectly reasonable to come to that conclusion based on your age, current health status and risk tolerance.
If you have perfectly healthy kidneys in your 90s, SGLT2i most likely won’t be ueseful to you. If you expect your bodily functions to eventually decline in your 60s-70s, you can extrapolate from existing clinical trials and figure that SGLT2i will have a protective effect on your kidneys.
![]() Egyptian paper…
Egyptian paper… ![]()
Chronic stress is recognized as a risk factor for neurodegeneration. Sodium glucose co-transporter 2 receptors (SGLT2) have been found in various brain regions, suggesting the potential neuroprotective properties of SGLT2 inhibitors as dapagliflozin (DGF). This study aimed to investigate the effect of DGF on behavioral, and neurodegenerative changes in chronic restraint stress (CRS) as an animal model of cognitive impairment. Forty-eight male rats were allocated into four groups: Control; CRS-subjected group, rats were subjected to chronic restraint stress for 6 weeks to induce cognitive impairment; DGF-treated CRS group, dapagliflozin was given daily by oral gavage; and DGF-administered group. Behavioral tests were performed and fasting serum glucose, insulin, and corticosterone levels were measured. Hippocampal oxidative markers, insulin signaling, mitochondrial function, amyloid beta, p-tau, and brain-derived neurotrophic factor (BDNF) gene expression were evaluated. DGF significantly prevented CRS-induced cognitive dysfunction (Y maze and Morris water maze tests). Also, DGF ameliorated hippocampal neurodegenerative changes by decreasing tau and amyloid beta levels, while increasing BDNF gene expression. DGF reduced hippocampal phosphorylated mammalian target of rapamycin (p-mTOR) and protein kinase B (p-Akt) levels. In addition to its antioxidant effects, DGF increased ATP levels and cytochrome C oxidase activity. These findings were confirmed by transmission electron microscopic (TEM) examination. The current study demonstrates a biological link between chronic stress, insulin resistance, and cognitive impairment. Dapagliflozin has therapeutic potential in alleviating cognitive deficits and neurodegeneration primarily due to its insulin-sensitizing and antioxidant properties, along with its capacity to enhance mitochondrial function.
So we have a BBB-crossing mTOR inhibitor @John_Hemming?
Not a single drug is approved for longevity so of course Consensus can’t recommend anything.
By the way, can we please stop sharing copy-paste of random AIs that don’t cite their source? It doesn’t have any value to our discussions.
Fair enough. But in this case I was sharing it only to get a better understanding of why it’s wrong