Clinical Pharmacokinetics of Sirolimus
“Variations in drug clearance and absorption rates result in a wide range of sirolimus Cmin ss values among patients receiving the same dose. In a pivotal phase III study (Global 302) among 212 patients receiving 2 mg/day, the Cmin ss over the 6-month period was 2.34 to 31.30 μg/L; the values for patients on 5 mg/day were 5.95 to 50.90 μg/L.”
This study reference was 2mg/DAY, constant exposure. If you’re taking say 5 mg/WEEK, it would not be too surprising to have a blood level 1 week later << 2 ug/L, given half life and n=1 kinetics.
Massive inter-person variation. Without checking your sirolimus blood levels, you have no idea what your dose is doing.
As well:
“When sirolimus is used in addition to cyclosporin and prednisone, a therapeutic concentration range of 5 to 15 μg/L is associated with protection from acute rejection episodes and adverse effects.”
By adverse effects, I believe they mean level 3/4 DLT: hypertriglyceridaemia (>300 mg/dl), thrombocytopenia, (<100 000/mm3) and leucopenia (<4000/mm3).
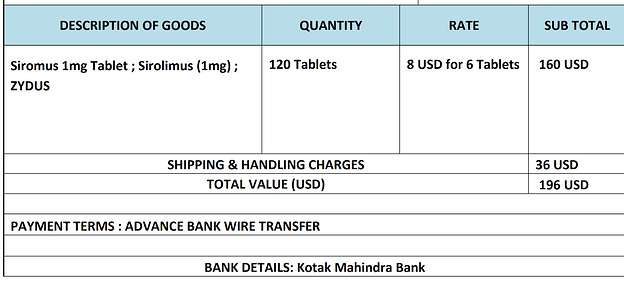
What site from India did you use? Cost? Thanks!
I currently buying Zydus rapamycin from an Indian drug exporter.
Niba Health Care
Just send an email to: Jagdish Nikose, rlhealthcare1928@gmail.com
requesting a quote for the amount you want.
Then give him your information and you will receive an invoice and bank information for the money transfer.
You need to set up an account with someone that can transfer money to Niba Healthcare.
I use Wise:
This is actually not complicated after you do it for the first time.
If you need more help just ask.
Thanks. That’s very helpful.
This guy from Varun Medical is quoting me $6 for a strip of 6 Zydus.
There’s a pharmacist in Little Neck , NY who charges $575 for 100 of the 2 mg dose from Dr. Reddy. Looks just like rapamune but generic. He’d have to ship it and pharmacies don’t generally do that.
Yes, I have had cheaper quotes from other Indian suppliers, but I have done several transactions and I feel very comfortable with him because he has always delivered with no hassle. He also has supplied me with other pharmaceuticals. Also, the guy lives in India and I don’t mind if he gets a little bit more in his pocket.
Just, like brick-and-mortar stores, we sometimes shop where we are comfortable and enjoy the experience even if they are not the cheapest place to shop.
my experience so far, Niba healthcare is the best. One other indian pharma sent me Zydus sirolimus pills that show expiration date this coming August.
That’s very true. I’ll go with NIBA.
Yes - I’ve also received sirolimus from companies where the expiration date is a few months from the date I got the drug. I really don’t worry about that at all. Sure - I’d prefer if the expiration date is a year or more in advance of the date I get it, but its not a huge factor in my decision making process on the different resellers.
What they found from the study is 90% of more than 100 drugs, both prescription and over-the-counter, were perfectly good to use even 15 years after the expiration date.
Do you set up the Wise money transfer as an ACH? Or as a wire transfer? Thank you!
Our experience with compounded, brand name, and generic sirolimus is quite different.
We tested several people on Rapamune and generic sirolimus and got nearly identical 24hr serum sirolimus levels. So there is no need to spend 2-4x on Rapamune.
As for compounded rapa, our experience show that its not as well absorbed as commercial rapa, but certainly not zero. One just needs to take a higher dose in order to get the same sirolmus serum levels (well, at least the 24hr level)
AgelessRx is planning to publish our findings once we exceed a certain minimum threshold of samples where we can have better confidence in our numbers - stay tuned…
Thanks for the update and perspective from AgelessRX!
By the way, when will we see any preliminary results from the PEARL study?
The rapa bioavailability data is separate from our PEARL trial. We started checking sirolimus levels on our PEARL participants, but then quickly discovered that this could lead to unblinding so we stopped.
Our last PEARL participant is scheduled to complete their 12 months in about 3 months. It will probably take us 2-3mo more to collect and analyze all the data. So we are targeting a mid-2024 publication date. We might make the data available sooner, though.
Hello desertshores - thank you for this excellent explanation of your buying practice - would I still be able to use a similar transaction from Canada?? Thanks again !!
I am currently using Maulik Parekh as a source, and Biocon Rapacan is the rapamycin I am using. My personal experience is that his packages clear customs faster than any other provider that I have used.
I have no idea how Canadian customs works. My thinking is, if I occasionally have a shipment seized, I am still saving a lot of money.
Click on this link for details
“Importing Rapamycin to Save Money (2) - #232 by desertshores”
Thanks very much for your help and information. I just ordered some from Maulik a week or so ago. i have a return email from him which includes a tracking number. Here’s hoping that Canadian Customs is not too sticky, thanks again!!