.
Note that this is a preprint.
It has not yet been peer reviewed by a journal.
.
ABSTRACT
Advanced Glycation End Products (AGEs) are the end result of the irreversible, non-enzymatic glycation of proteins by reducing sugars. These chemical modifications accumulate with age and have been associated with various age-related and diabetic complications. AGEs predominantly accumulate on proteins with slow turnover rates, of which collagen is a prime example. Glycation has been associated with tissue stiffening and reduced collagen fibril remodelling. In this study, we investigate the effects of glycation on the stability of type I collagen, its molecular-level mechanics and its ability to perform its physiological role of self-assembly. Collagen AGEing is induced in vitro by incubation with ribose. We confirm and assess glycation using fluorescence measurements and changes in collagen’s electrophoretic mobility. Susceptibility to trypsin digestion and circular dichroism (CD) spectroscopy are used to probe changes in collagen’s triple helical stability, revealing decreased stability due to glycation. Atomic Force Microscopy (AFM) imaging is used to quantify how AGEing affects collagen flexibility, where we find molecular-scale stiffening. Finally we use microscopy to show that glycated collagen molecules are unable to self-assemble into fibrils. These findings shed light on the molecular mechanisms underlying AGE-induced tissue changes, offering insight into how glycation modifies protein structure and stability.
.
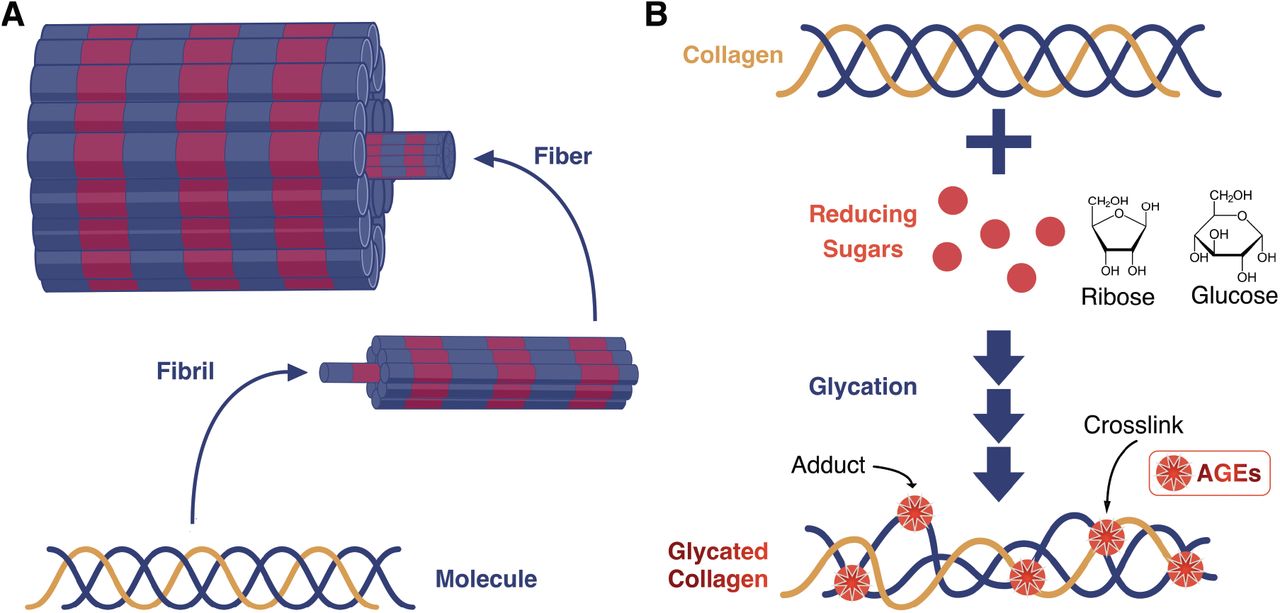
Collagen structure and glycation. (A) Visual representation of different structural levels of collagen type I organization. Banding is meant to represent gap and overlap regions found in assembled fibrils and is not to scale. Type I collagen is comprised of two identical α 1 (blue) chains and one α 2 (yellow) chain. (B) Glycation of collagen. Sugars including ribose and glucose can participate in glycation. Reactive amino groups found on lysine and arginine residues commonly participate in AGE formation. AGEs can modify a single site to form an adduct or two adjacent sites to form a crosslink.
.
FOR THE REST OF THE STUDY, PLEASE VISIT THE SOURCE HERE
.