From the Age1 email newsletter today:
A guide to extend longevity by delaying menopause
Refusing menopause as an inevitable truth
Reproductive aging is largely overlooked in the longevity field
My mom started perimenopause in 2023. As she experienced headaches, night sweats, hot flashes, and brain fog, she would tell me she didn’t feel like herself. As she started hormone replacement therapy (HRT) – the only treatment for menopause – I took a deep dive into this world. I was already in the field of longevity, but menopause wasn’t something I often saw discussed, which puzzled me.
The life expectancy for women in the U.S. is around 80 years, while the average menopause age is 51. As medicine continues to develop and life expectancy increases, the number of years post-menopause will continue to increase . Despite this fact, reproductive aging is significantly under-researched. Only 10.8% of the NIH’s 2020 budget went to women’s health research, and reproductive biology and aging are largely siloed fields. However, menopause could be an important lever we can pull to extend years of healthy life in women , and I hope more longevity researchers will start considering the field of reproductive aging.
This post will consider how longevity and menopause might be connected, their implications, and possible approaches to delaying menopause.
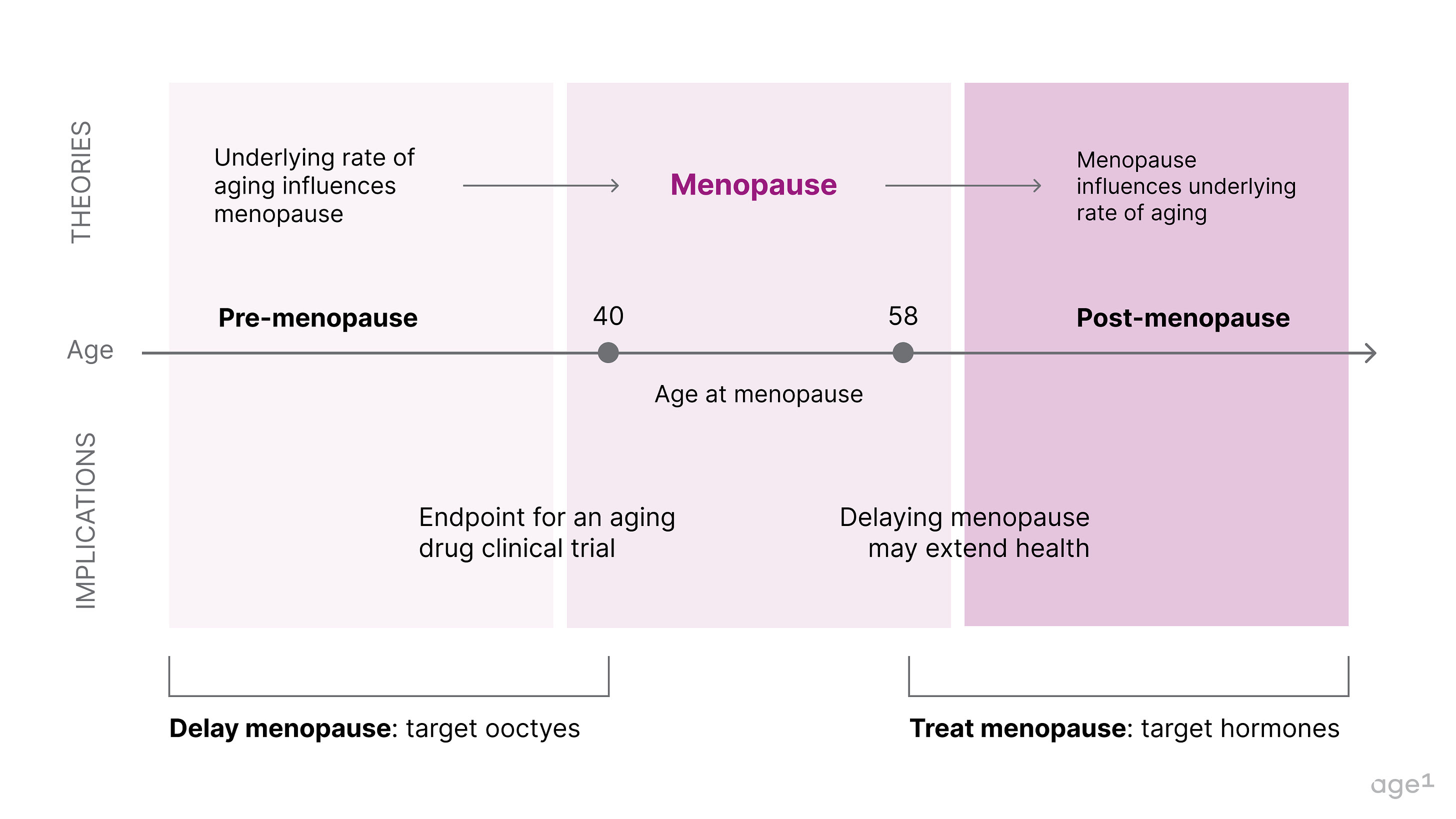
Fig 1. Post at a glance
Table of contents:
- Women have a finite pool of oocytes
- Menopause is about fertility but also quality of life
- Two non-mutually exclusive theories could explain the connection between menopause and aging
- Theory 1: Healthy aging can influence the age of menopause
- Theory 1 implication: menopause could be an indication in clinical trials for “repair” aging drugs
- Theory 2: Menopause directly impairs health
- Theory 2 implication: the body is a network, and estrogen is a major node to impact overall health
- How to delay menopause
- Conclusion
- Appendix: Interventions that extend fertility and protect the ovarian reserve
Women have a finite pool of oocytes
While men continue to produce sperm throughout their lives, women are born with all the eggs they will ever have. These eggs are part of the primordial follicle pool and remain immature oocytes until puberty. It’s pretty mindblowing that the egg cell that made you was produced by your mother when she was a developing fetus in your grandmother!
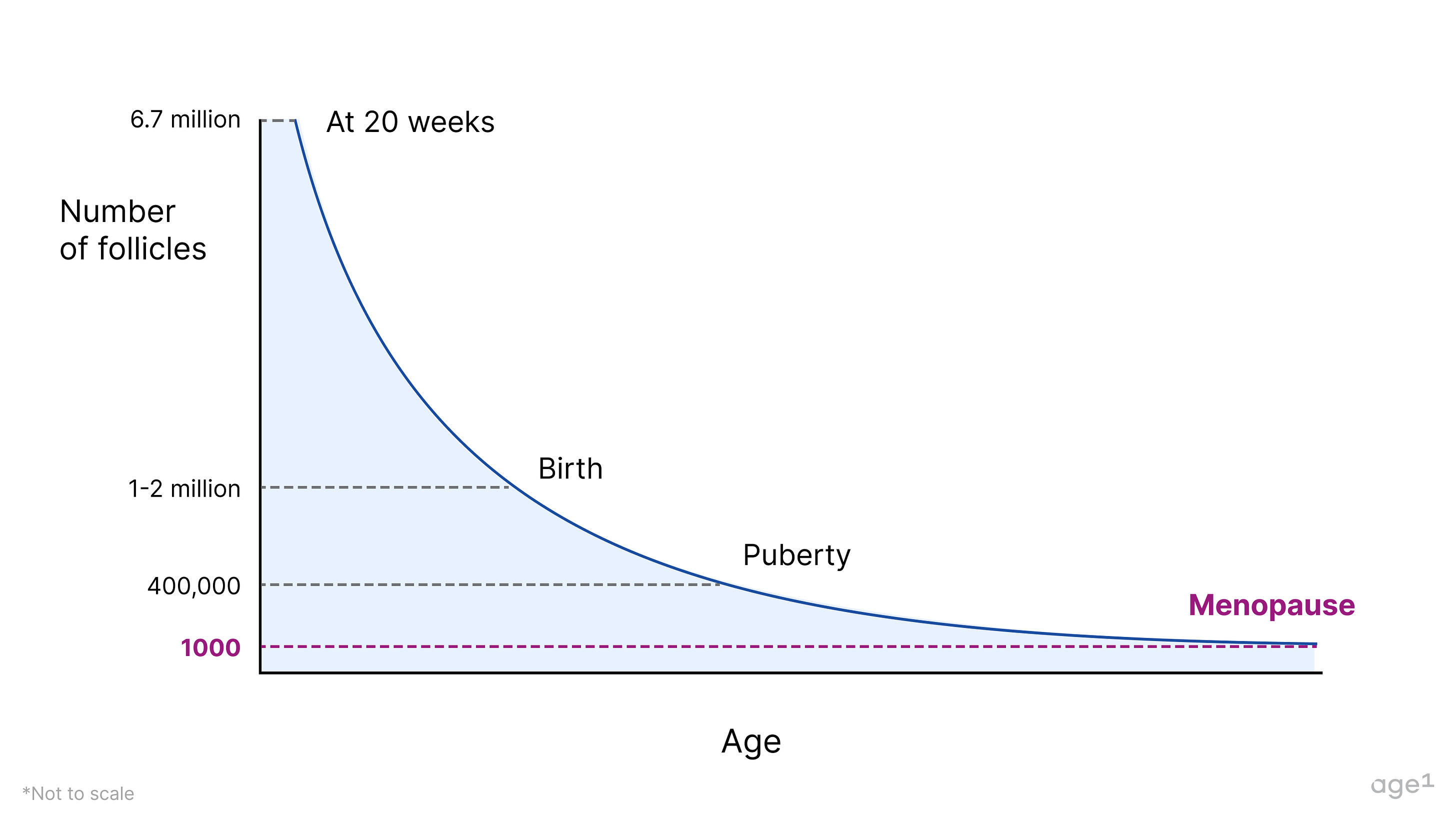
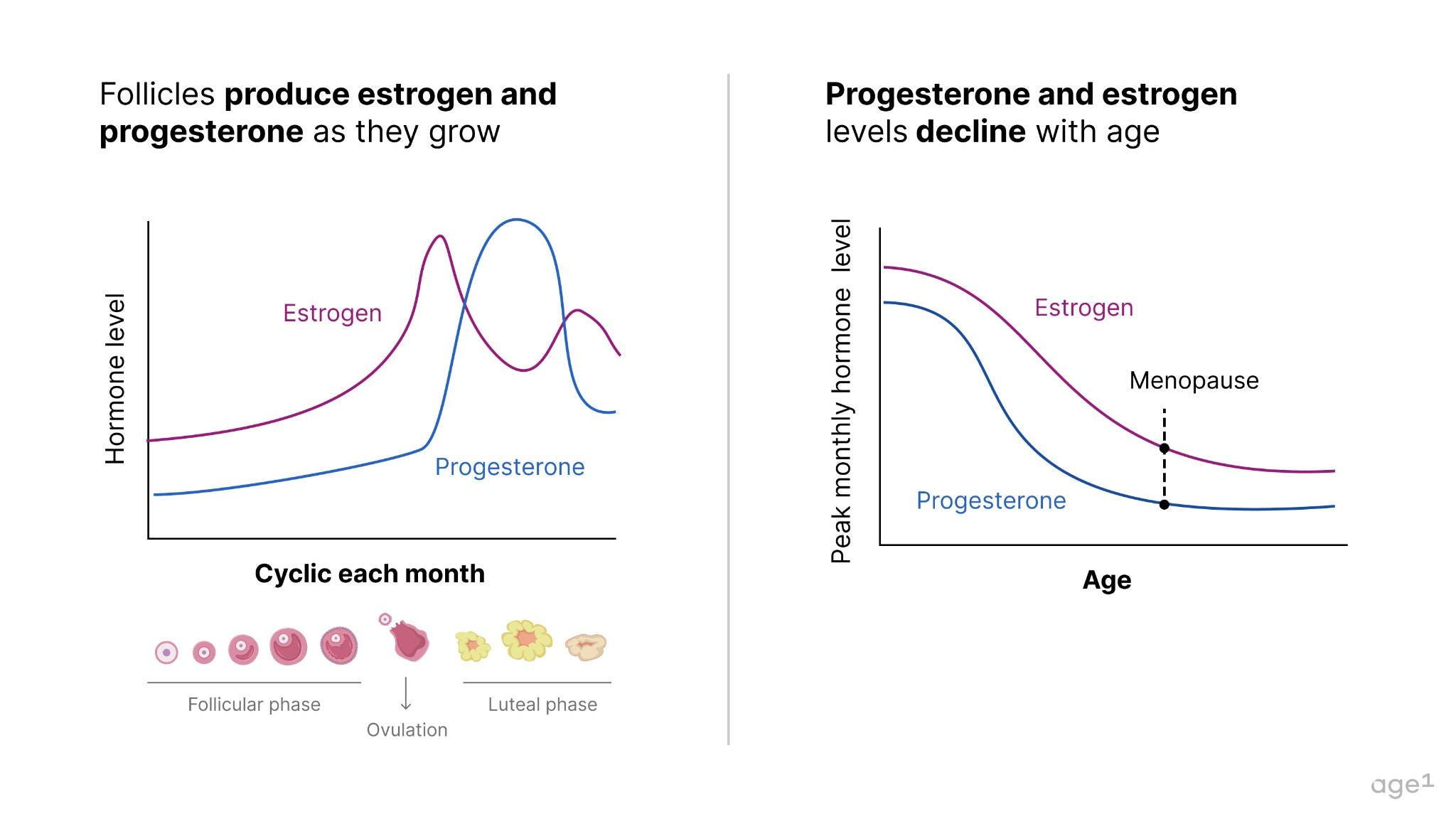
A female fetus typically has around 6–7 million oocytes at 20 weeks of gestation, dropping to 1–2 million at birth. When puberty begins, she only has about 400,000 oocytes, meaning she’s already lost over 90% of the oocytes she started with . During much of her adult lifetime, about 1,000immature oocytes will be activated every month to start maturation; and only one mature oocyte will be ovulated. This egg, if fertilized by a sperm, will become a baby 9 months later. The cells surrounding oocytes – granulosa and theca cells – form follicles , which produce important hormones for health, specifically progesterone and estrogen . With a decline in the number (as well as quality!) of oocytes, these hormones also decline with age .
Although it is not clear how many follicles a woman has at menopause, that number is expected to be around 1,000. The oocytes left are of poor quality and can’t grow to become mature eggs. At this point, a woman becomes unable to have children, and it is said that the ovarian reserve is depleted .
Fig 2. The number of follicles declines with age
Menopause is about fertility but also quality of life
Menopause is, in part, about fertility and the autonomy to decide when to start a family. If you’re a woman and you’d like to be a mother, you are also most likely familiar with the biological clock ticking in the background of your decisions. Initially faint in one’s 20s, this tick grows louder each year, reminding you that your fertility is declining with age. From dating decisions to major career options, this ticking is subtle but present.
The chances of having a natural pregnancy decline in a woman’s early 30s, when she still has plenty of immature eggs left, but the general trend in developed nations is that women are having children at a later age.
However, menopause is also about quality of life . Along with menopause comes a wave of unfavorable symptoms – hot flashes, insomnia, and mood swings. Not surprisingly, menopausal women have lower quality-of-life scores compared to pre-menopausal women.
Other health effects pertain to underlying health and aging. With the depletion of the ovarian reserve, the ovaries no longer produce much estrogen and progesterone – hormones that are key to various biological functions. This begs the question - how are menopause and aging connected?
Fig 3. Follicles produce estrogen and progesterone. Since follicles decline with age, so do estrogen and progesterone.
Two non-mutually exclusive theories could explain the connection between menopause and aging
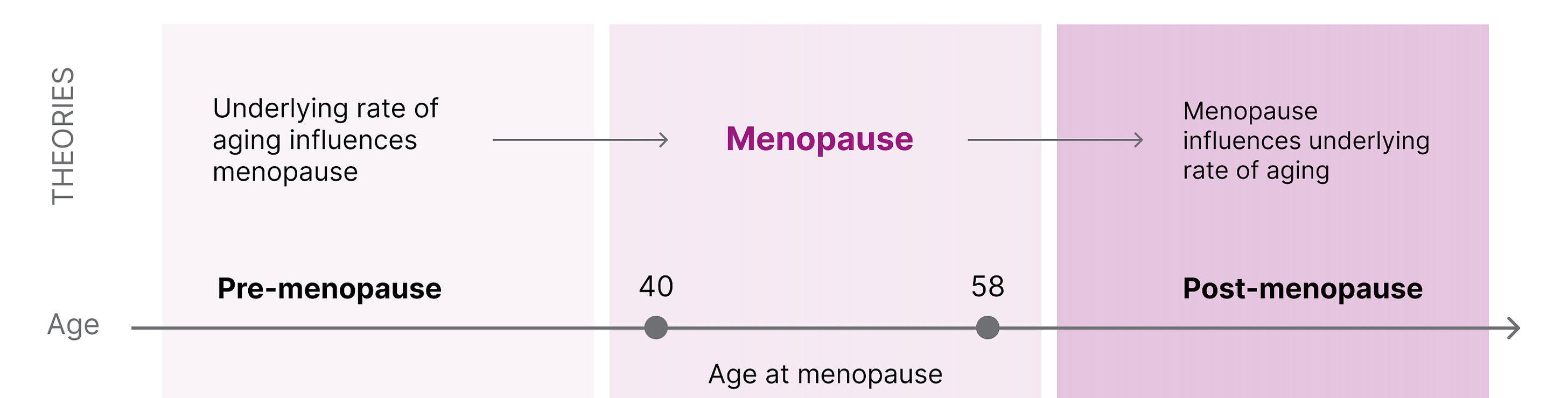
Later menopause is associated with longer survival, and centenarian women are four times more likely to have children in their 40s compared to women who survived only to 73. However, it is unclear if menopause causes more biological aging or if the age of menopause correlates with the underlying rate of aging . There are two reasonable hypotheses that are not necessarily mutually exclusive:
-
Healthy aging → Delayed menopause
-
An underlying biology of aging dictates the age of menopause . This suggests that if one has accelerated aging, this would be reflected in early menopause. If slow aging, then later menopause.
-
Delaying menopause → Healthy aging
-
Menopause can accelerate the underlying biology of aging . This suggests that menopause (to some extent) causes aging and that if menopause were artificially delayed, there would be less significant physiological aging.
Fig 4. The connection between menopause and underlying rate of aging.
Theory 1: Healthy aging can influence the onset of menopause
Women who are in worse health by various metrics, such as type 2 diabetes and heart disease, are at risk of earlier menopause. For example, higher total cholesterol levels and blood pressure in pre-menopause years are associated with an earlier age of menopause. This suggests that one’s health status before menopause can indeed dictate the age of menopause.
If menopause is a marker of underlying aging, women who undergo menopause earlier are aging at a faster rate than women who experience menopause at a later age. It then becomes possible that some longevity interventions will also delay menopause if they are administered early enough. So, even if a drug is not developed with the intent to delay menopause, it could. For example, calorie restriction and rapamycin consistently extend lifespan and prolong reproductive span (the period that female mice can have offspring).
Theory 1 implication: menopause could be an indication in clinical trials for “repair” aging drugs
If some aging drugs have the potential to delay menopause, one exciting implication is that menopause could be an indication for a clinical trial. This, however, heavily depends on the type of aging drug.
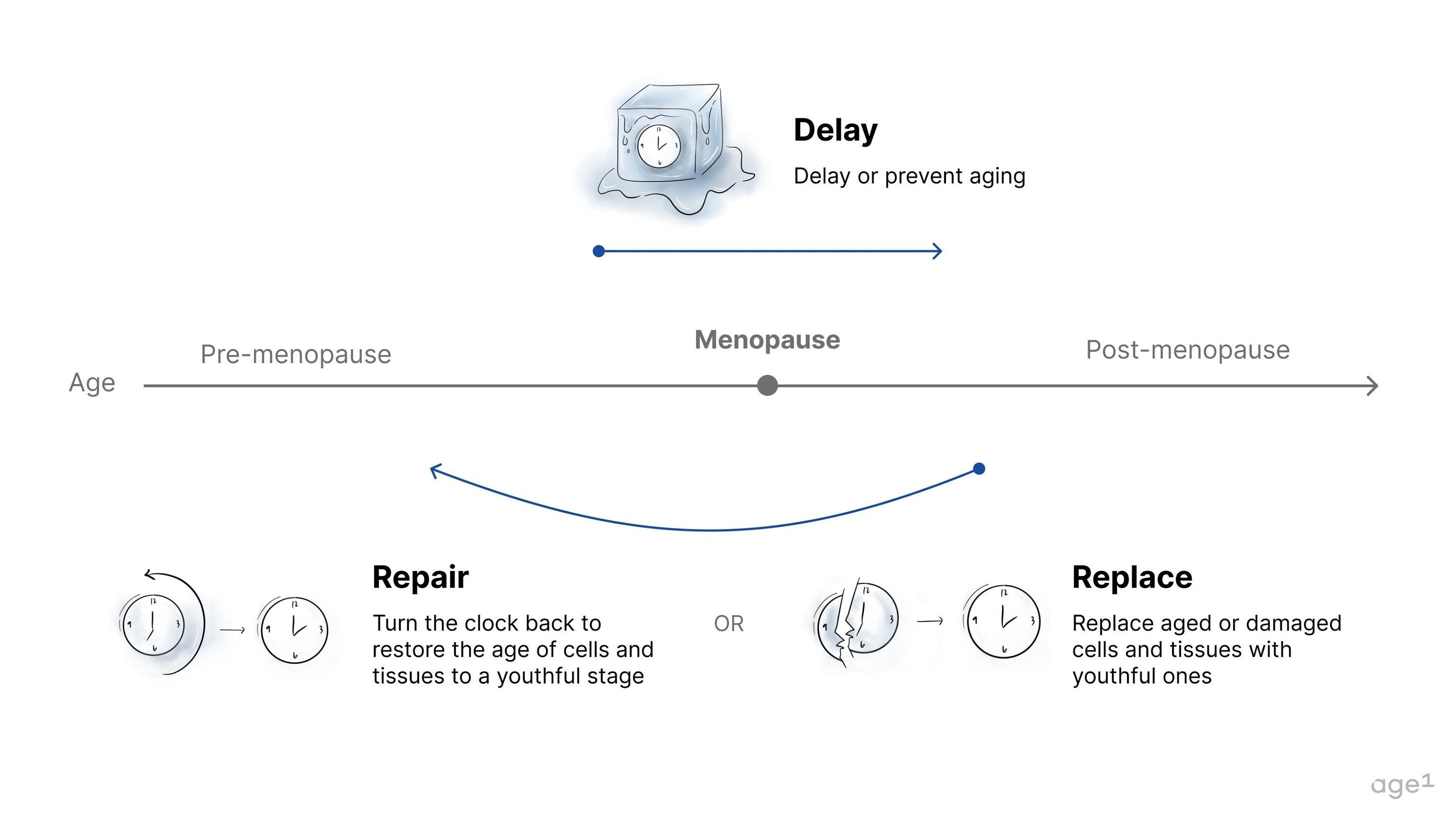
The three paradigms of aging drugs are delay, repair, and replace. Specifically, drugs that delay aging may be the primary candidates also to delay menopause. These have the potential to delay the decline in overall health markers, which could, in turn, delay aging in the ovaries. If administered before the onset of menopause, they may delay menopause entirely. In contrast, interventions that repair or replace damaged tissue would likely be administered after the onset of menopause, when damage has accumulated. Unless the ovaries are directly targeted, repairing or replacing damaged tissues in the body would not affect an aged ovary with few oocytes and low levels of hormone production.
If menopause is a marker for biological aging, there’s a potential it could be used as an endpoint for aging drugs, which allows for the design of a relatively straightforward clinical trial. For instance, Columbia University is currently sponsoring a clinical trial studying the effects of Rapamycin on perimenopause.
Overall, it seems that the age of menopause is strongly connected to underlying biological aging, and there’s even the potential that a drug for aging might delay menopause. But instead of just being a marker of aging, could changing the age of menopause be a lever to extend healthy years of life that women enjoy?
Theory 2: Menopause directly impairs health
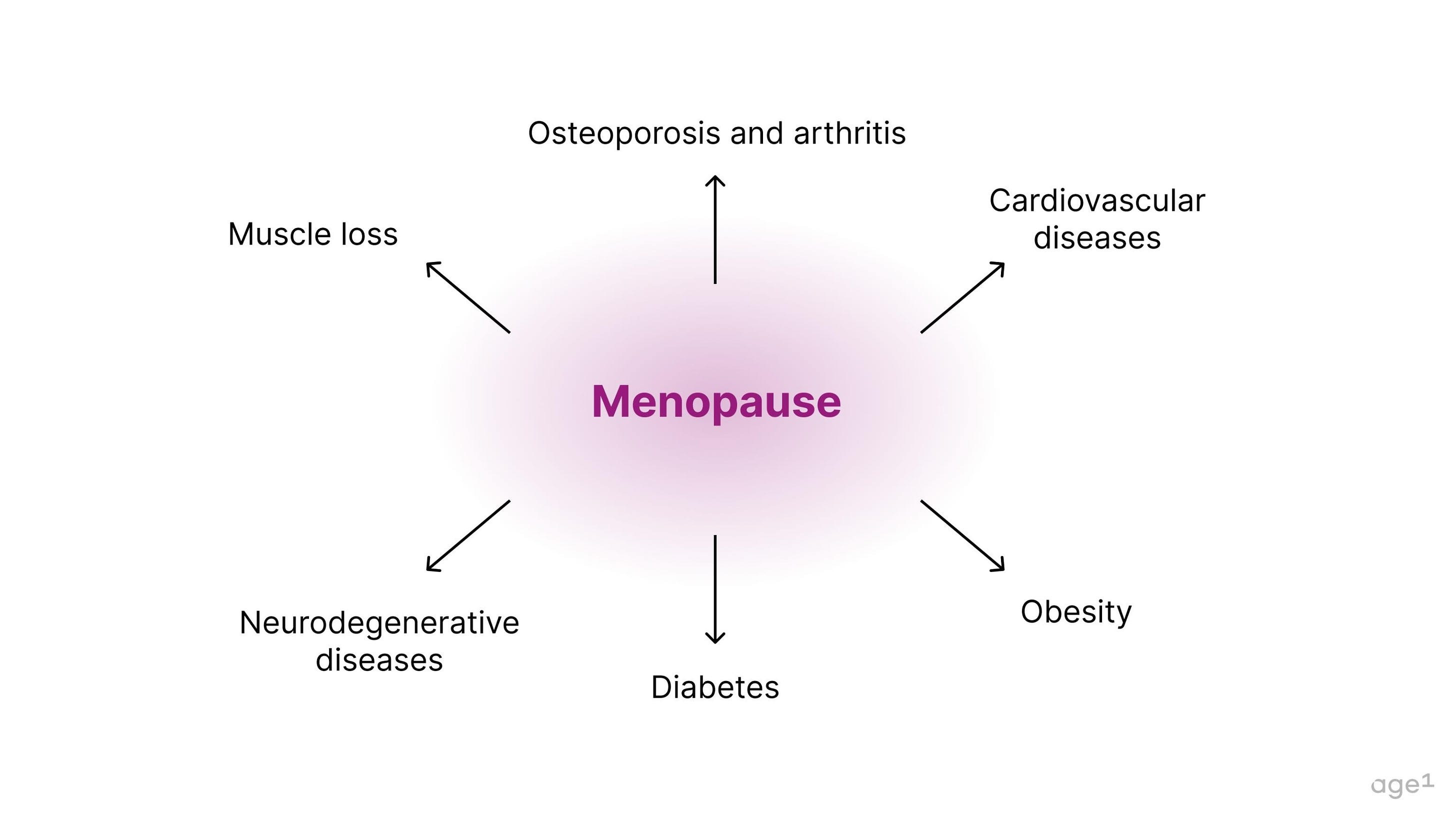
Menopause is connected to a variety of age-related diseases, including muscle loss, osteoporosis, arthritis, cardiovascular disease, obesity, diabetes, and neurodegenerative diseases. Specifically, the deficiency in estrogen post-menopause seems to contribute to this decline in health .
Fig 6. Menopause is connected to a variety of age-related diseases.
Importantly, rodents don’t have the same hormonal profile as menopausal women. Even as they age, some will still have sustained estradiol levels, which doesn’t recapitulate low human levels. A standard model system to study menopause includes mice that have been ovariectomized – the ovaries are surgically removed, mimicking the human inactive ovaries after menopause. The effect of ovariectomy, as well as the role of estrogen in various age-related diseases, are discussed next:
Cardiovascular disease
At a glance: estrogen supports cardiovascular health .
Numerous studies have suggested that the risk of cardiovascular diseases rises after menopause. Also, compared to men, women have lower blood pressure until they reach their 60s. Estrogen might be a significant culprit in this decline in cardiovascular health.
For example, some studies have shown that estrogen can prevent cardiac fibrosis by blocking the fibroblast to myofibroblast transition. In animal models of cardiovascular disease, young females have lower rates of vascular injury, a slower progression to heart failure, and lower mortality than males. However, these differences can be reduced or eliminated by estrogen deficiency or removal of the ovaries. This suggests that the higher estrogen levels produced by the ovaries in females than in males might be a protective factor.
At a molecular level, estrogen significantly inhibits the expression of adhesion molecules in endothelial cells, which play a significant role in the development of atherosclerosis. It also substantially influences vascular injury response through increased nitric oxide production.
In humans, studies have suggested that the risk of cardiovascular mortality is higher for women with early menopause than for those with late menopause. This aligns with the hypothesis that the longer women are exposed to estrogen produced by the ovaries, the better. Hormone replacement therapy also decreases the risk of cardiovascular disease when started within 10 years of menopause onset (although such results are controversial).
These studies suggest that the reduction in estrogen removes protective female mechanisms that support cardiovascular health.
Obesity
At a glance: estrogen is protective against obesity .
More men are overweight than women. Similarly, male mice have a higher susceptibility to obesity than female mice, suggesting that this gap might be partly due to biology and not just environmental factors. Removing the ovaries in female mice eliminates the protection against obesity, and estrogen supplementation restores this protection. Ovariectomized mice on a high-fat diet are also protected against obesity when administered estrogen. This indicates that estrogen from the ovaries is a major player in this protection against obesity.
In humans, a study following healthy women for 4 years determined that all women gained subcutaneous abdominal fat over time; however, only postmenopausal women had a significant increase in visceral fat, known as “toxic fat.” Also, obesity and metabolic syndrome happen more oftenpostmenopause than before. Estrogen deficiency is likely a major causal factor that increases the risk of obesity with age in the case of women.
Diabetes
At a glance: estrogen regulates glucose .
A similar pattern to that observed in cardiovascular disease is also seen with diabetes. There are more diabetic males before puberty but more diabetic females after the age of menopause. Research has suggested that estrogen regulates glucose homeostasis, which explains why pre-menopausal women have a low incidence of type 2 diabetes compared to age-matched men. In animal studies, ovariectomized mice have higher fasting blood glucose, serum insulin, triglycerides, and LDL cholesterol than mice with functioning ovaries.
This indicates that estrogen deficiency post-menopause is likely a causal factor leading to diabetes in old age, which, in turn, leads to a decline in general health and increased risk of various age-related diseases.
Neurodegenerative diseases
At a glance: estrogen has neuroprotective effects .
Undoubtedly, menopause comes with significant changes to the brain. Early age of menopause is associated with an increased risk of dementia, and multiple studies show that postmenopausal women have less grey matter in the brain compared to pre-menopausal women.
The question becomes how much of the changes seen in the brain are due to estrogen deficiency (and thus menopause itself) or other aspects of aging. Although the numbers vary in the literature, some studies suggest that women have lower stroke incidence until the late 50s and early 60s, when they catch up to men. In animal models, ovariectomized mice do worse in maze learning, and estrogen recovers reference memory. Similarly, ovariectomized rhesus monkeys have impaired cognitive function, which is improved with estrogen replacement. These studies suggest that while estrogen deficiency doesn’t directly cause neurodegenerative diseases, it likely has neuroprotective effects that can improve cognition and prevent (to some extent) neurodegeneration.
The neuroprotective effects of estrogen have been widely reported. For example, ovariectomized mice that are treated with estrogen have more new cells in the hypothalamic region compared to mice without the treatment. Ovariectomized mice treated with specifically an estrogen receptor alpha agonist have improved learning. Also, estrogen has been shown to contribute to ischemic neuroprotein in mice and has been strongly implicated in the prevention of Alzheimer’s. In humans, women on HRT are at lower risk of Alzheimer’s, and estrogen increases gray mattervolume in the hippocampus of postmenopausal women.
Although the specific molecular pathways involved in estrogen-mediated neuroprotection are not well elucidated, estrogen likely plays an essential role in maintaining proper cognitive function. In turn, its deficiency might make the brain more vulnerable to cognitive decline and neurodegenerative diseases.
Muscle loss
At a glance: ovarian hormones support muscle growth .
Loss of muscle strength happens in both males and females. In males, there is a more gradual process, and in females, there is a sharp decline around menopause. However, hormone replacement therapy (HRT) canprevent this decline of strength in women. One study compared monozygotic twins on and off HRT and observed that those who took the therapy had greater relative muscle area and smaller relative fat area.
In animal models, ovariectomized mice experience muscle atrophy in a time-dependent manner. Also, ovariectomized mice with induced muscle atrophy experience limited muscle regrowth unless supplemented with estrogen.
At a molecular level, estrogen helps with satellite cell activation and proliferation, which are crucial for muscle maintenance and restoration. In humans, some studies have suggested that testosterone and progesterone (which also decline with menopause), but not estrogen, stimulate muscle protein synthesis in women after menopause.
Although the connection between estrogen, progesterone, and muscle synthesis is hazy, menopause seems to cause a sharp decline in strength, which can be partly explained by the loss of ovarian hormones. This suggests another major biological function strongly connected to the ovaries.
Osteoporosis and arthritis
At a glance: estrogen deficiency causes osteoporosis
Estrogen promotes the activity of osteoblasts – cells that make new bone– and estrogen deficiency directly causes osteoporosis in both older men and postmenopausal women. Osteoporosis can dramatically decrease the quality of one’s life, and there are about 1.5 million osteoporotic fractures in the U.S. each year.
Similarly, estrogen deficiency has also been implicated in arthritis through the regulation of cartilage metabolism. It’s been reported that ovariectomized rats have increased cartilage turnover and surface erosion. Ovariectomized macaques with one ovary removed have similar symptoms. Various clinical studies have also shown that osteoarthritis is related to estrogen levels. Hormone replacement therapy can preserve and even increase BMD (bone mineral density) at all skeletal sites in postmenopausal women. Additionally, the administration of hormonal oral contraceptives can also restore bone turnover and normal bone density.
Estrogen deficiency directly causes osteoporosis, making postmenopausal women especially vulnerable to age-related frailty. It is also strongly associated with arthritis, indicating that menopause is a significant factor in decreasing the quality of life of older women.
Theory 2 implication: the body is a network, and estrogen is a major node to impact overall health
Estrogen signaling influences numerous systems in the body and seems to have protective effects that are lost with menopause. In this framework, estrogen deficiency is a domino piece that, when pushed over, impacts various downstream biological mechanisms that become more vulnerable and contribute to unhealthy aging.
Ovariectomies in both animal models and humans suggest damaged overall health. Ovariectomized dogs have shortened lifespans, and the same is seen in mice. (It’s noteworthy that while ovariectomized mice are a common model, these animals are also deficient for any other signals the ovaries would otherwise produce.) In humans, women who underwent ovariectomy before the age of 46 were more likely to develop depression, coronary artery disease, arthritis, asthma, osteoporosis, and chronic obstructive pulmonary disease, among others. Finally, mice who had their ovaries surgically replaced with younger ones experienced a 40% increase in life expectancy.
The first wave in women’s health that led to profound social changes entailed contraceptives, specifically the pill. The second wave in reproductive health will target ovarian aging and delay menopause to improve underlying health.
How to delay menopause
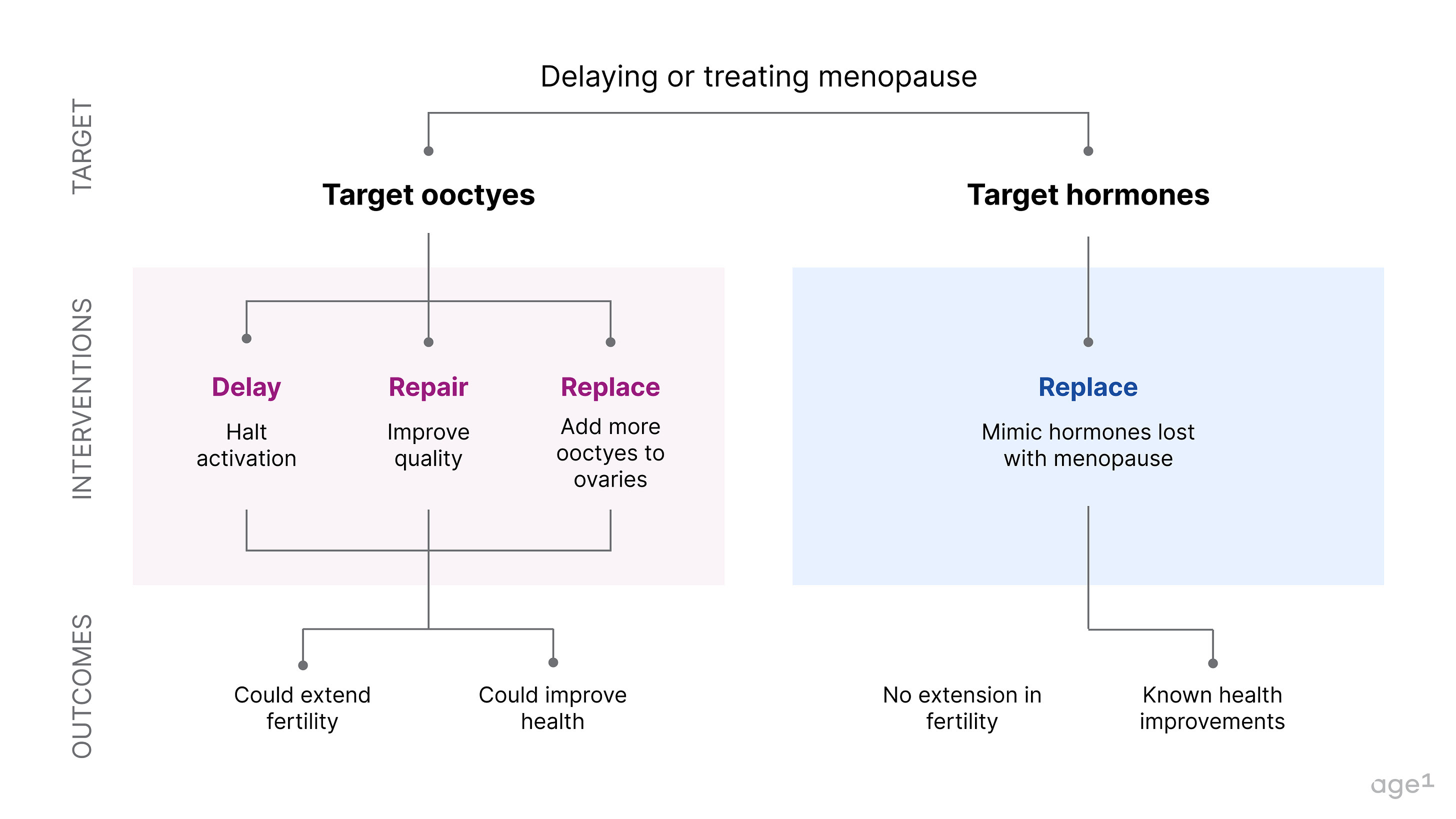
If menopause is a major node in the aging process that marks a pivotal moment in a cascade of health decline, then delaying it could significantly increase healthy years of life. This could be done by directly targeting oocytes. Alternatively, menopause could also be avoided by correctly mimicking hormonal signals.
Fig 7 . How to delay or treat menopause
Targeting oocytes to delay menopause
- Inhibiting activation (delay)
The trigger of menopause is the depletion of the ovarian reserve, so what if we could delay the loss of oocytes? One major way to do this is by halting their activation. The female body activates about 1,000 follicles each month to get 1 egg ovulated. If only 500 were activated, this would theoretically double the years women have between puberty and menopause. Various interventions that have been shown to extend fertility in mice work by inhibiting the activation of oocytes - such as rapamycin, AMH (anti-mullerian hormone), and even calorie restriction.
This approach focuses on the number of oocytes, but the ovarian environment undergoes physiological changes with age, negatively impacting their quality . For example, ovaries become more inflammatory and fibrotic with age. When considering extremes, it’s possible that a woman could have many oocytes at an old age due to their growth inhibition. Still, if oocytes are of bad quality, they might not produce hormones properly or be competent for fertilization.
Pro: most upstream cause of menopause
Con: quality of oocytes might be a limitation
- Targeting oocyte quality (repair)
Another approach to protect oocytes is to target their quality directly. There are likely various ways to do this, but one potential option is to increase DNA damage repair. Oocytes in the ovaries accumulate mutations with age, and as the ovaries become more inflammatory and fibrotic, the oocytes experience more DNA damage. Oocytes that cannot grow due to an improper environment or poor quality go through cell death and are called atretic. More DNA damage means more oocytes become unviable, leading to quicker depletion. More substantial DNA damage repair could protect oocyte quality and prevent atresia.
Age at menopause is highly heritable, and its associated genes in humans are strongly implicated in DNA repair. This is consistent with the fact that old populations also have enhanced DNA repair activity.
Pro: ensures that oocytes are competent for fertilization
Con: even if oocytes are repaired, they will still be lost in monthly cycles
- Providing more oocytes (replace)
If the trigger of menopause is the depletion of high-quality oocytes in the ovaries, these could be theoretically replaced. Although replacing an entire ovary with a younger one is unfeasible, there’s a potential that sections of the ovaries could be frozen at a young age and re-implanted later. This is called ovarian tissue cryopreservation and is done in prepubertal patients undergoing chemotherapy. These individuals can’t undergo egg freezing and are likely to have their oocytes damaged during the chemotherapy treatment. Instead, they can have strips of their ovaries cryopreserved and then surgically re-implanted as adults. In the context of delaying menopause, this is unpreferable due to two surgeries. It is also unclear how much of an effect small sections of the ovaries would have in delaying menopause.
Another potential way to replace oocytes is by making new ones ex vivo. An adult cell, such as a blood or skin cell, could be taken and then transformed into an induced pluripotent stem cell (iPSC). We have yet to learn how to make these into oocytes, but various groups are working on it. If this becomes possible, these could be potentially re-implanted into the ovaries. The quality of the ovaries would still be a concern, as they would need to provide a proper environment for the oocytes.
Pro: unlimited supply of oocytes
Con: unclear if reimplanted oocytes would produce proper hormones in an aged environment
Regardless of how oocytes are protected, the ovaries must also be considered an environment to house the oocytes. How to prevent inflammation and fibrosis will be significant questions that need to be answered for an effective strategy to delay menopause.
Targeting hormonal signals to treat menopause
Alternatively, instead of focusing on the oocytes that produce the hormones, we could focus on the hormones themselves. While this wouldn’t extend fertility, it could reverse the health decline due to menopause. Currently, menopausal women are given HRT (hormone replacement therapy), which usually is composed of estrogen and progesterone. While this undoubtedly helps with menopause symptoms, it doesn’t reverse its effects on the body entirely.
There’s little research in this area due to the lack of model organisms that recapitulate human menopause, which makes it challenging to develop these interventions. Two specific reasons for the inefficacy of HRT include the difficulty in dosage optimization and the decline in estrogen receptors with age. Estrogen and progesterone in a woman pre-menopause are tightly regulated in cycles. However, hormone replacement therapy gives a constant dosage (in the case of patches, for example) or a spike dosage (in the case of oral pills). Additionally, the expression of estrogen receptors also declines with age, so even if estrogen is available, receptors might not be functional in cells at their proper levels.
A better understanding of how to mimic endogenous hormones more closely, as well as their signaling through receptors, could provide better treatment for menopause and reverse its effects on underlying health.
Pro: The number or quality of oocytes doesn’t matter
Con: Downstream effect of menopause, and difficult to perform research due to the lack of model systems that recapitulate menopause
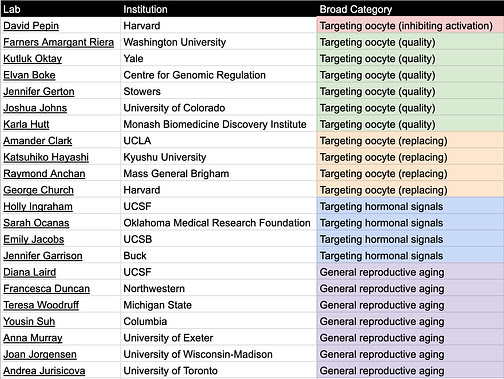
Table 1. Relevant academic labs categorized by delay/treat menopause category
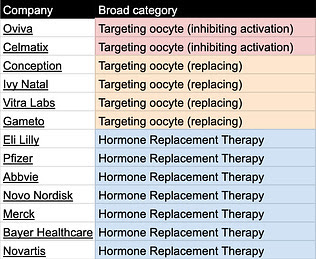
Table 2. Relevant companies categorized by delay/ treat menopause category
Tables can be found here and will be continuously updated. If you know of other relevant labs/companies email me at carol[at]age1.com.
Conclusion
Menopause is a significant node that can be influenced to improve numerous other systems in the body and ensure healthy aging. Delaying menopause will be one of the first steps to achieving a world where women can live healthily and actively, regardless of an arbitrary chronological age.
Unfortunately, reproductive aging is significantly under-researched, and within biotech, although 50% of the population experiences menopause, there are only a handful of companies focused on reproductive longevity. As aging biology becomes mainstream, the aging of the reproductive system and its effects on overall health must be carefully considered to enable longer, healthier lives.
I want to create a future where my grandmother feels healthy and active. Where my mother has full autonomy to do all the things that she loves and isn’t limited by physical weakness or mental constraints. A world where I have the choice to have children when it feels right and don’t need to sacrifice my career because of a biological clock. Whatever your reasons are, I hope you’ll join me in building this future.
Appendix
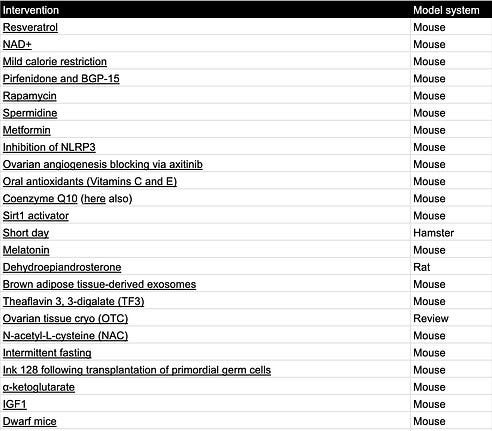
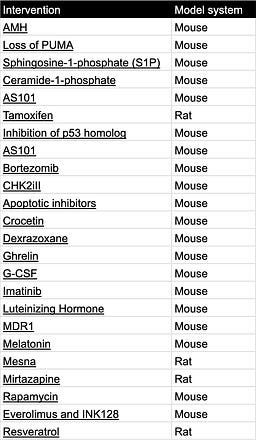
Tables can be found here and will be continuously updated. If you know of other interventions, email me at carol[at]age1.com.
Interventions that extend fertility in rodents:
Table 3. Fertility extending interventions
Interventions that protect the ovarian reserve when rodents are treated with chemotherapy:
Table 4. Ovarian reserve protecting interventions