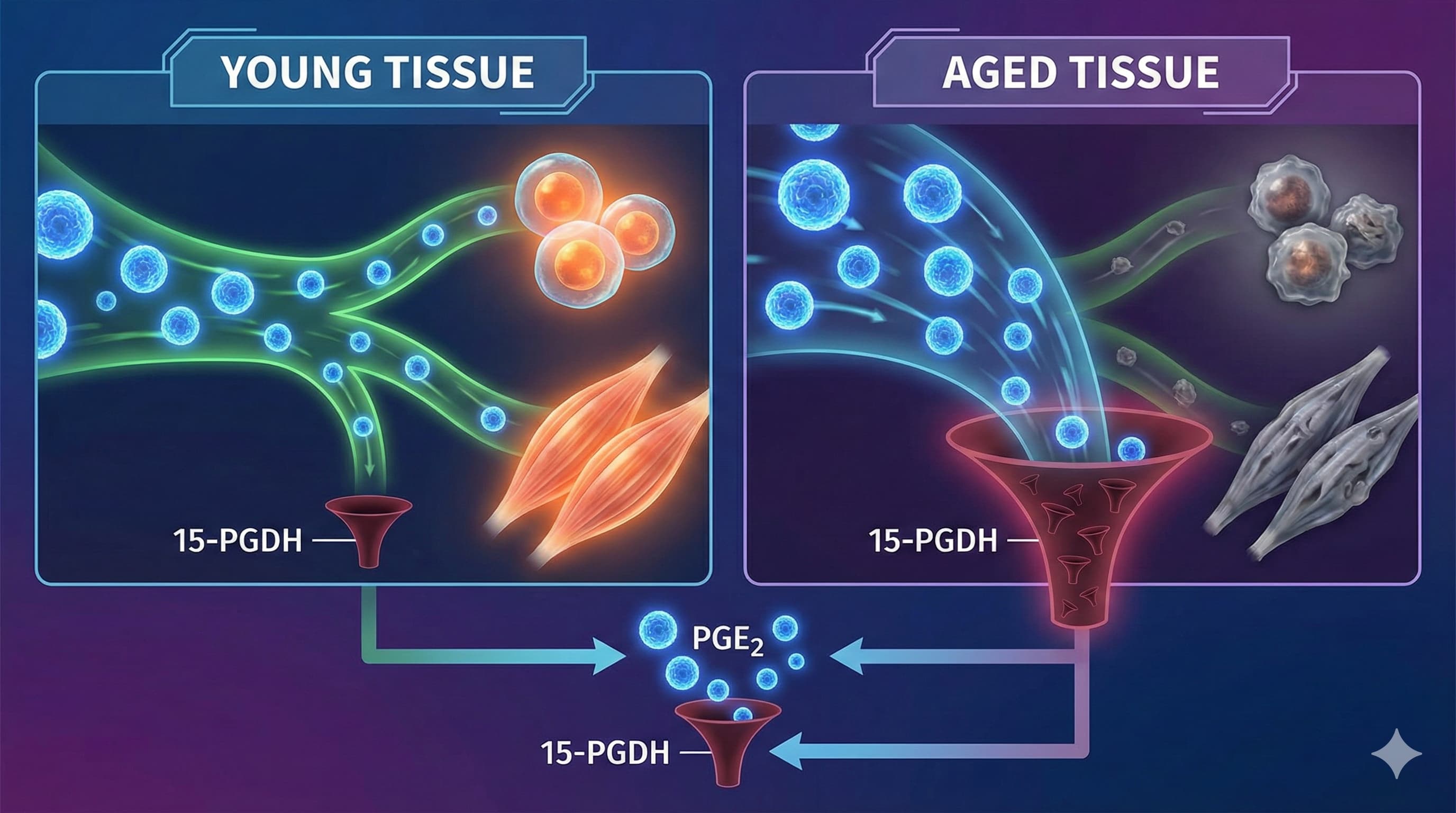
A new paradigm in longevity science has emerged from the lab of Dr. Helen Blau at Stanford: the identification of “gerozymes”—enzymes that actively degrade youth-preserving molecules as we age. This review consolidates a series of breakthrough findings centered on one specific culprit: 15-prostaglandin dehydrogenase (15-PGDH).
The “Big Idea” is that aging is not just a failure of production, but an acceleration of destruction. 15-PGDH acts as a metabolic drain, aggressively breaking down Prostaglandin E2 (PGE2), a critical signaling molecule essential for stem cell function, muscle repair, and mitochondrial health. In young tissues, 15-PGDH levels are low, allowing PGE2 to orchestrate repair. In aged tissues (muscle, brain, cartilage), 15-PGDH levels skyrocket, depleting PGE2 and locking cells into a senescent, dysfunctional state.
Blocking this enzyme with a small molecule (SW033291 or the clinical candidate MF-300) achieves systemic rejuvenation. The intervention restores withered muscle mass and strength Inhibition of 15-PGDH rejuvenates aged muscle (2021), tightens the leaking blood-brain barrier to improve cognition Inhibiting 15-PGDH blocks blood–brain barrier deterioration (2025), and—in a breakthrough late-2025 finding—regenerates hyaline cartilage in osteoarthritic joints Inhibition of 15-PGDH promotes cartilage regeneration (2025). This is not just slowing decline; it is the functional reversal of sarcopenia, neurovascular breakdown, and osteoarthritis by “plugging the drain” on the body’s own repair signals.
Source:
- Open Access Narrative Review Paper: From Cell Reprogramming to Tissue Rejuvenation: Countering Aging by Targeting a Gerozyme
- Date: Volume 66, 2026
- Institution: Stanford University School of Medicine (USA) Journal: Annual Review of Pharmacology and Toxicology Impact Evaluation: The impact score of this journal is 13.1 (2024), evaluated against a typical high-end range of 0–60+ for top general science; therefore, this is a High impact journal (ranking within the top tier of the Pharmacology & Toxicology category).
Related Reading:
- The Culprit of Aging - Helen Blau's work at Stanford / PGE2, 15-PDGH
- Longevity Summit 2025 Reporting - Helen Blau PGDH Presentation
Part 2: The Biohacker Analysis
Study Design Specifications
- Type: Review Article summarizing multiple In vivo (Murine) and In vitro (Human Ex Vivo) primary studies.
-
Subjects:
- Species: Mice (Mus musculus).
- Strain: C57BL/6 (Standard wild-type background).
- Age Groups: Young (2–4 months) vs. Aged (24 months). Note: 24 months is widely accepted as “old” but not “geriatric.”
- Human Data: Ex vivo analysis of human muscle myotubes and osteoarthritic cartilage samples.
-
Lifespan Analysis:
- Findings: The primary papers reviewed here (Science 2021, Science 2026) focused on Healthspan (grip strength, treadmill endurance, cartilage thickness) rather than maximum lifespan extension.
- Control Verification: The “900-Day Rule” (Pabis et al., 2023) warns that interventions often look successful only because control groups die prematurely (<800 days). The C57BL/6 controls in Blau’s studies were healthy at 24 months (~730 days). While not a full longevity study, the healthspan data is robust because the treatment reversed established frailty rather than just delaying death. It is crucial to note that 15-PGDH knockout mice are reported to live a “normal lifespan,” suggesting this pathway compresses morbidity rather than extending maximum life 15-PGDH inhibitors for tissue repair (2017).
Mechanistic Deep Dive
-
The Pathway: PGE2 / EP4 Receptor Axis.
- Young State: High PGE2 → Binds EP4 Receptor → cAMP/CREB signaling → Mitochondrial biogenesis (via PGC1 alpha) + TGF-β suppression.
- Aged State: High 15-PGDH → Low PGE2 → Mitochondrial dysfunction + TGF-β fibrosis.
-
Organ Priorities:
- Skeletal Muscle: Reverses sarcopenia by restoring mitochondrial geometry and function.
- Joints: Promotes hyaline cartilage regeneration (the “good” cartilage) over fibrocartilage (scar tissue).
- Brain: Tightens the Blood-Brain Barrier (BBB) and reduces neuroinflammation, independent of amyloid plaques.
Novelty
The paper flips the script on inflammation. Biohackers typically view Prostaglandin E2 (PGE2) as “bad” (pro-inflammatory, pain-causing) and use NSAIDs to block it. This research proves that physiological levels of PGE2 are mandatory for regeneration. The problem in aging is too little PGE2 in local stem cell niches, not too much. It introduces “Gerozymes” as a new target class: enzymes that are upregulated with age and must be inhibited (unlike sirtuins, which we try to activate).
Critical Limitations
- The “NSAID Paradox”: The intervention increases PGE2. Chronic high PGE2 is a known driver of pain and, critically, tumorigenesis (colon cancer). The study claims safety, but long-term systemic elevation of PGE2 in humans carries a theoretical cancer risk that short-term mouse studies may miss.
- Translational Gap: Mice have different metabolic rates and prostaglandin handling than humans. The “rejuvenation” seen in mice is rapid (1 month); human repair cycles are slower.
- Route of Administration: The mouse studies often use intraperitoneal (IP) injection. Oral bioavailability of second-generation inhibitors (MF-300) is claimed but data is proprietary.