It seems most likely that its both actually. To remove old hazardous stuff in the blood cannot simulate adding young blood factors and adding young blood factors alone cannot simulate clearing away junk.
Yes, I’ve always intuitively believed this, and my research kept coming up with so many beneficial benefits. Conceptually, I’ve been most attracted to interventions that impact every cell in the body…diet, exercise, blood donation. Pharma is often a narrow singular pathway, although Rapamycin targets a central longevity nutrient sensor pathway, so is perhaps unique (and supported by a plethora of longevity studies)
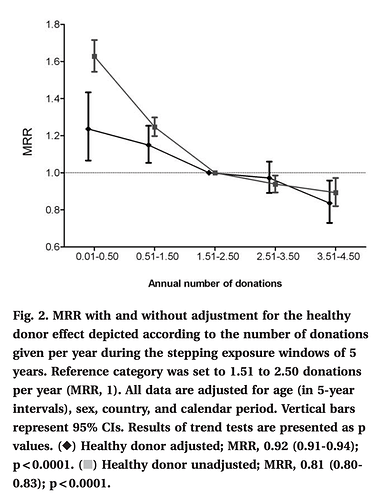
Blood donation and blood donor mortality after adjustment for a healthy donor effect
https://sci-hub.se/https://doi.org/10.1111/trf.13205
“We observed an inverse relationship between donation frequency and mortality. Analyses adjusted only for demographic characteristics showed a 18.6% reduction in mortality per additional annual donation (95% confidence interval [CI], 16.8%-20.4%). After additional adjustment for the internal healthy donor effect, each additional annual donation was associated with a 7.5% decreased mortality risk 7.5%”
I donate 9x/year +/- for over 5 yrs…extrapolate the MRR (mortality rate ratio) curve?!
“According to several studies, blood donation is associated with better health outcomes. A 33–
88% lower risk of cardiovascular disease (CVD) has been reported in donors versus non-donors. Repeated blood donations reduce blood viscosity, thereby potentially lowering blood pressure and the risk of plaque rupture. It is also known that with a 500ml whole blood donation, up to 250mg of heme iron is lost. This iron loss, which may reduce oxidative stress and the availability of iron for malignant cells, may have a protective effect against insulin resistance and atherosclerosis, and may as well protect against cancer.”
Data mining of human plasma proteins generates a multitude of highly predictive aging clocks that reflect different aspects of aging
Could be as simple as rejuvenating the proteome.
hmm. The body will gravitate to a homeostasis in albumin qty (proteome) needed. Like most of the human systems, things are tightly regulated. In the mouse study, they were extremely careful to replace exactly the quantity for this reason. Of course, there are certain diseases that cause high or low albumin, but these are not phlebotomy induced.
Secondly, they’d never let you donate very 7 weeks if there was an issue with increasing/decreasing albumin levels.
Exercise, eat less (and low protein) in a narrow window, and donate blood regularly…Rapamycin is neutered? ![]()
So to summarize, blood donations lower iron and blood viscosity as well as rejuvenating the proteome, of which albumin is probably the most important factor. There may be more benefits that we don’t know about.
Of interest also is the role of rapamycin in this. Hematopoietic stem cells are a necessary part of this replacement and studies have shown that HSC’s are rejuvenated by rapamycin. Can’t leave that out of the picture or stem cells may not be able to keep up.
Ah - the rapamycin angle comes up. This study suggests how rapamycin seems to prevent hematopoietic stem cell aging / enlargement, but I wonder if (because of mTORC1 inhibition) we might want to do blood donations when our rapamycin levels are low, so that the “new albumin creation” can proceed without any hinderance by low mTORC1?
Hmmm. That’s something that we need to think about.
There are other benefits without a doubt. Let me dig up my archive and share.
But what if donations are a hormetic stressor that supports blood stem cell rejuvenation? I assume bone marrow is signalled/marshalled to support in phlebotomy. HSC anti-senescence (preventing exhaustion)?
From the paper reference, Rapamycin prevents enlargement of HSC. Blood donation will trigger new small stem cells from bone marrow, thus allowing Rapamycin to act in its HSC therapeutic modality keeping NEW stem cells from enlarging.
In the lifespan.io article it mentions (as I think everyone knows):
Over the currently normal human lifetime, they decrease in quality through the accumulation of damage, as seen in the other Hallmarks of Aging, and, eventually, the pool of stem cells runs out.
Given the Hayflick limit, shouldn’t increasing blood donations also result in increased stem cell turn over, and thus moving towards exhausting the pool of stem cells?
HSC’s have the ability to self regenerate but rapamycin augments that ability.
https://stemcellsjournals.onlinelibrary.wiley.com/doi/pdf/10.1002/stem.3313
Maybe there’s a limit on the number of donations per year that would be ideal without risking stem cell exhaustion.
Hormesis almost certainly plays a role in this but it may be possible, like other hormetic stressors, to overdo it.
Duly noted Hayflick limit, although it’s only a theory.
“What gives more hope is that no one has actually proved that the Hayflick limit actually limits the lifespan of an organism. Correlation is not causation. For instance, despite having a very small Hayflick limit, mouse cells typically divide indefinitely when grown in standard laboratory conditions. They behave as if they have no Hayflick limit at all when grown in the concentration of oxygen that they experience in the living animal (3-5% versus 20%). They [make enough telomerase] an enzyme that replaces degraded telomeres with new ones. So it might be that currently the Hayflick “limit” is more the Hayflick “clock”, giving readout of the age of the cell rather than driving the cell to death.
The same site reference:
“the pool of stem cells can regenerate itself”
They only reference exhaustion = SASP (senescence)
I am betting donation is a fundamental rejuvenation intervention, preventing senescence in HSC, like Rapamycin…up to some limit of course.
BUT…will monitor closely my CBC for derailment?
@rivasp12 Is CBC a good tracker of this stem pool dyanmic/derangement?
Check in with me at 100. ![]()
RapAdmin
To your point
So you have significant telomere attrition of 30 base pairs per year in your stem cells leading to genome instability and mutations. These mutated stem cells are the predominant forms after age 70. Not good.
Maybe a combination of rapamycin for stem cell rejuvenation and Centella Asiatica for telomere maintenance as well as a sweet spot for blood donations would be smart.
Anemia may be a good aging phenotype to monitor for age related impacts on hematopoietic stem cells
Reached out to the blood donation site, asked about stem cell exhaustion with long term donors. This is the response:
“There is no indication of stem cell exhaustion in donors, thanks for your ongoing donations,
Mindy Goldman MD”
Interesting new research:
Full Paper:
It’s always comes back to mitochondria…
“Mechanistic investigations reveal that young sEVs stimulate PGC-1α expression in vitro and in vivo through their miRNA cargoes, thereby improving mitochondrial functions and mitigating mitochondrial deficits in aged tissues. Overall, this study demonstrates that young sEVs reverse degenerative changes and age-related dysfunction, at least in part, by stimulating PGC-1α expression and enhancing mitochondrial energy metabolism.”
In vitro heterochronic parabiosis identifies a multi-tissue geroprotective factor from young blood
Yousin Suh, Columbia University, USA, presents at the 11th Aging Research and Drug Discovery meeting
Some of the post-docs are starting a company (Perpetual Bio) based on this research, and I searched online for the website or other info, but they don’t appeared to have put anything up yet. @invivo this would seem to be an investment opportunity for you guys to look into.
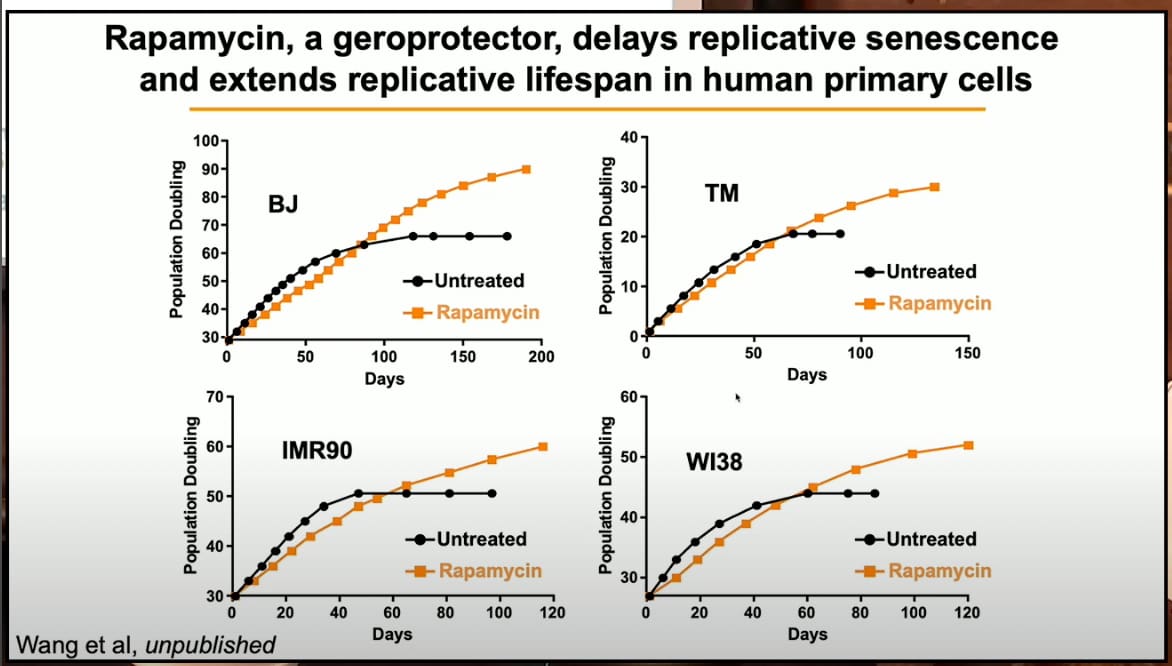
Also, an interesting slide on rapamycin in her presentation:
Thanks for posting. FYI the rejuvenation factor they mention in the video is Pigment epithelium-derived factor (PEDF) also known as serpin F1 (SERPINF1).