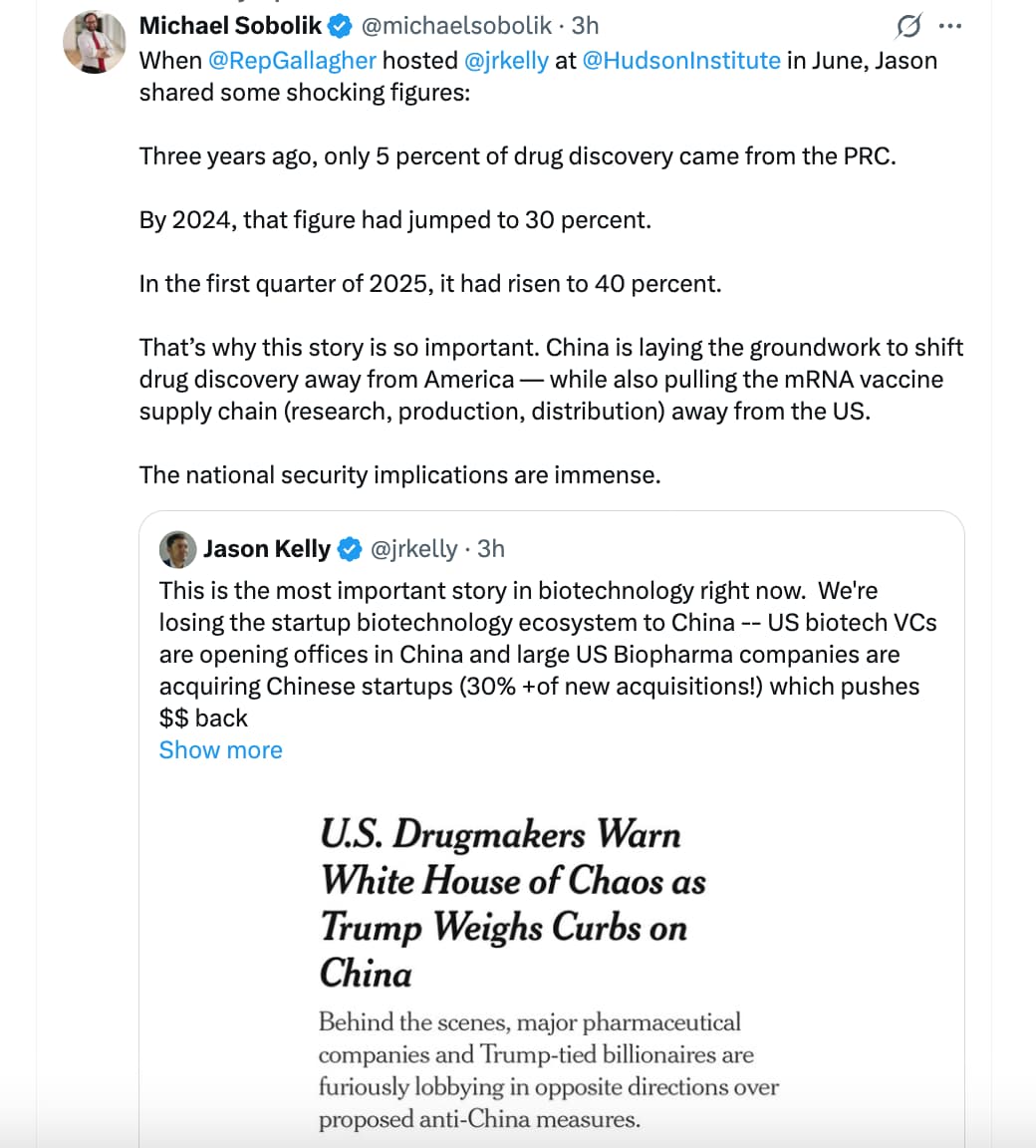
White House officials are working on a draft executive order, which, if enacted, could impose harsh restrictions on experimental treatments discovered in China that leading drugmakers have relied on extensively to boost their pipelines, The New York Times reported on Wednesday.
Smaller biotechs with links to China, such as I-Mab (IMAB), BeOne Medicines (ONC), and Zai Lab (ZLAB) traded sharply lower in reaction.
Billionaire investors and top company executives with close ties to the Trump administration have reportedly initiated behind-the-scenes lobbying efforts with both opposing and supportive views on the matter as administration officials seek feedback on the order, according to the report.
Venture capitalist Peter Thiel, Google (GOOG) (GOOGL) co-founder Sergey Brin, and other influential personnel holding relatively illiquid investments in U.S. biotech startups have argued for a crackdown, four people familiar with their lobbying efforts said.
On the other spectrum are some of the leading pharma companies, such as Pfizer (NYSE:PFE) and AstraZeneca (NASDAQ:AZN), which have relied on licensing deals to obtain low-cost experimental drugs from China to replenish their pipelines.
A White House spokesperson denied that the administration was “actively considering” the order, adding “safeguarding our national and economic security is a top priority for the administration.”
The draft order calls for several measures to crack down on China-invented drugs, including a proposal to increase scrutiny on deals that U.S. drugmakers have so far signed without any restrictions when buying rights for Chinese medicines.
Per the draft order, such deals are set to undergo a “mandatory review” by a U.S. national security committee called the Committee on Foreign Investment in the United States. The order also proposes measures to discourage the use of clinical trial data generated from patients in China and calls for a more rigorous review from the FDA, as well as higher regulatory fees.
The report comes at a time when the biotech dealmaking activity between Western pharmaceutical companies and Chinese biotechs has increased in recent years.
Major drugmakers such as AbbVie (NYSE:ABBV) and Merck (NYSE:MRK) have in-licensed nearly a third of their external molecules from Chinese biotechs in 2024, up from 10%–12% in 2020–2022, according to a report from Stifel in January.
Roche (OTCQX:RHHBY) (OTCQX:RHHBF), Novartis (NVS) (OTCPK:NVSEF), AbbVie (NYSE:ABBV), and GSK (GSK) were among other major pharma companies to strike licensing or buyout deals with Chinese companies to strengthen their drug pipelines last year.