Did he state the dose for such a protocol?
No he didn’t. We will have to wait for the results.
Brad’s fairly young… so that I could see that make sense.
For 60 years up… I think more frequently would be in order. I do 6 mg weekly. Pretty much stuck to that the past 5 years… no breaks… gone as high as 12 mg every 10 days and low as 4 mg weekly. But always come back to my sweet spot 6 mg weekly. Actually feel off at high dose and like age is creeping up faster at low dose - skin quality dryer… sinus less healthy.
My son started up again after a 2 year break… he is young… 33 years… he does 4 mg weekly. Helped him first time reset weight point… lost 25 pounds.
I am curious to see how second round goes.
Trouble is younger folks benefits are not as obvious as when one is older… functional declines… body changes.
The young feel great… all the more reason to take rapamycin early and hover at your younger self a few decades.
It’s difficult to assess. If you take rapamycin at too high doses or too frequently, you risk infection. If you take it too infrequently or at too low a dose, you won’t see any beneficial effect beyond placebo.
The mice trials point to high/very high doses producing the most lifespan extending effect, somewhat independent of frequency. But rapamycin in mice chow has a different bioavailability than rapamune pills. And gender-specific differences are not seen in humans either.
Anywhere from 5mg once weekly to 20mg once every other week has case reports of being somewhat safe. But it may depend on where you live, where you work etc. I wouldn’t touch rapamycin if I worked as a RN or as a kindergartner, for example.
I think Brad’s Rapamycin trial was for people who are older, so I would imagine that his advice would be based on that age population. I’ll wait for the full results before dissecting his interpretation though.
As people know I am a high dose infrequent person.
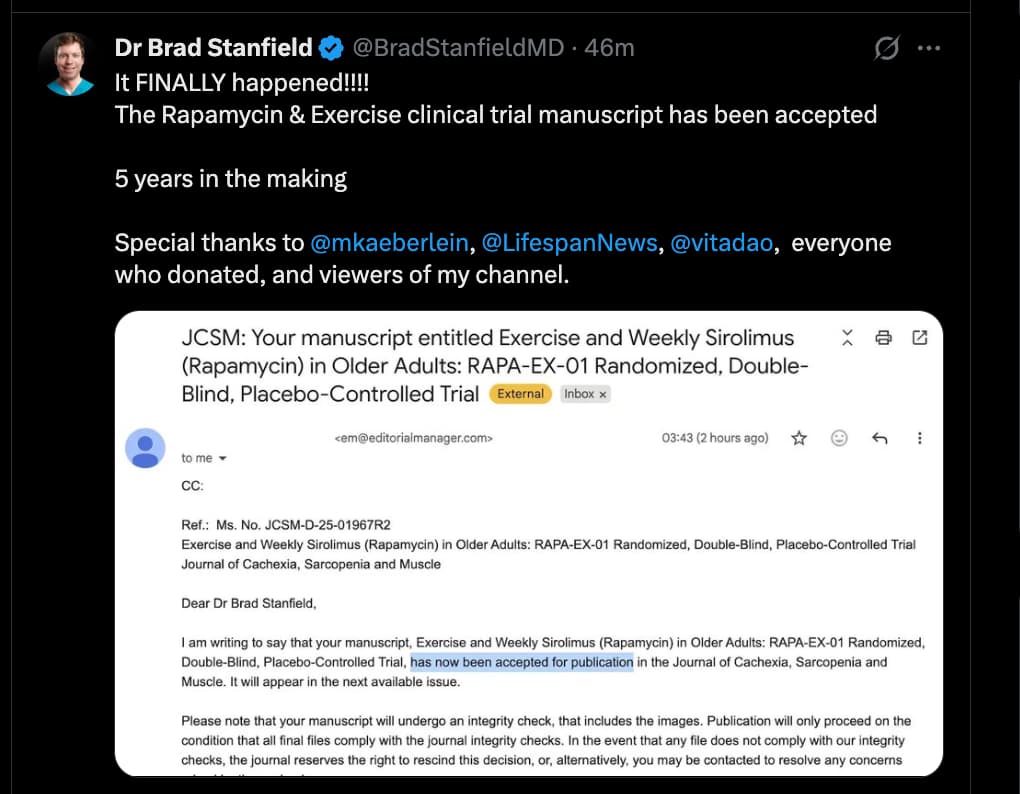
Its interesting… the original protocol in his study was 6mg/week as outlined here: Rapamycin Exercise Study Moves Forward: Dr. Brad Stanfield's Study Registered in NZ
I’m not sure how he could determine that once every 4 to 8 weeks would be better given this study.
Yes I’m confused as well but I won’t rush to judgement. Brad always has a pretty compelling explanation so I’m eager to hear why he thinks that.
@John_Hemming maybe you were ahead of the curve after all
The trough is an important period.
Since I started late (age 81), I have been following Dr. Blagosklonny’s advice from his papers and Twitter—the highest tolerable weekly dose without unpleasant side effects.
As an aside, IMO: There is no proof that a weekly high dose of rapamycin significantly suppresses mTORC2. I have experienced slightly slower wound and mosquito bite healing, but not enough to concern me.
Unlike most, I titrated down from doses that were causing me to have diarrhea.
The maximum dose I could take was 16 mg with olive oil without causing diarrhea.
So for the last ~4 years, I have been taking this dose, or the equivalent, as I am now using GFJ to extend my supply of rapamycin. Up until recently, I was taking 6 mg weekly with GFJ.
One of the reasons for the highest tolerable dose is the theory that if a sufficiently high dose is administered, some of it will cross the blood-brain barrier and exert a therapeutic effect.
For the last 4 weeks, I have titrated up to 8 mg weekly with GFJ. I also encapsulate my rapamycin tablets in enteric-coated capsules and take them with GFJ and olive oil.
I don’t know how much, if any, effect the enteric-coated capsules make.
Apparently, my body has adjusted to taking high-dose rapamycin because I am experiencing no side effects, except that I always feel great for the next couple of days.
Results: Over the last four years, I have not contracted a cold, the flu, or COVID-19. Never experienced an infection. I am pain-free, which I find remarkable, because my contemporaries are always complaining about this and that pain. Rapamycin has dramatically improved my condition of chronic actinic keratoses.
Initially, I experienced an elevation in lipids and glucose levels, but Brillo (bempedoic acid with ezetimibe and metformin) addressed that.
I plan on titrating up to 10 mg weekly with GFJ/enteric-coated capsules/EVOO.
As for Dr. Stanfield’s study, it is too small and too short for me to be interested in the results, which I find predictable. But I appreciate his effort. If I wait for the weekly high-dose trial, the train will have already passed.
I don’t advocate high-dose rapamycin for anyone, especially younger people, where it is not needed. But for those of us at an advanced age, it is one of the very few probable life extenders.
Interesting perspective.
There’s also this idea that a weekly dose can actually improve your immune system (the 2014 Mannick paper). Theoretically weekly doses will help to clear away crappy immune cells.
Personally, I am 39 and I am taking 2mg or 3mg per week. It’s definitely affected my Levine phenotypic age, knocking about 10 years off it (lowering RDW and WBC). I can’t say I noticed anything else. My thinking is that I am still young-ish, and nothing is really wrong and needs fixing yet. However, I can benefit from autophagy, particularly in rapidly dividing/produced cells like immune cells and RBCs.
When I was taking 8-10mg per week, my A1C mysteriously went higher to 5.5 (there could be another reason but that’s my educated guess). I took a little break and dropped it to 5mg per week and now my A1C is down to 4.9.
I think dosage is a key factor here and I was probably using too much.
Getting close to seeing the results!
Source: https://x.com/BradStanfieldMD/status/2027622566714831084?s=20
Me too. But wages are down, sewage is flowing into the ocean, and a lot of kiwis are moving to Australia.
What’s the reason for the move? Economics? When I visited both countries last year. Australia definitely seemed more economically vibrant than NZ.
Better wages, more housing, and a lower cost of living, as well as more sunny days.
Yes. That’s exactly what it seemed like.
OK, but NZ has a lot more vibrant greenery. Nothing in Australia is as green as some fields in NZ. NZ is almost on par with Ireland for the intensity of the green. Australia is a parched red hostile land (except the fabulous beaches ![]() ), by contrast NZ looks like a land of milk and honey. Of course appearances can be deceiving, but nobody can deny that NZ is spectacularly beautiful.
), by contrast NZ looks like a land of milk and honey. Of course appearances can be deceiving, but nobody can deny that NZ is spectacularly beautiful.
And I would love to live there, even though Jacinda Ardern, the former prime minister of New Zealand, has moved with her family to Australia.
Indeed. I was fortunate enough to visit NZ and it was the most gorgeous place I’ve ever seen. Still many places I haven’t been, though.
Out of curiosity, during my vacation I asked about moving there and was told you needed to be under a certain age and have a certain net worth so you wouldn’t be a drain on their system. In a quick AI search, it seems that was not exactly true. If seems if you are past the age of 55, you can buy your way in through large investments.