I buy this one. I get it when there’s a 40% off Swanson products. I’m on their email list and they’re always running some kind of promotion with varying % off, so I just wait until it’s 40% off; it happens about once a month.
https://www.swansonvitamins.com/p/swanson-ultra-deltagold-tocotrienols-50-mg-60-sgels
This one is double strength:
https://www.swansonvitamins.com/p/swanson-ultra-double-strength-tocotrienols-100-mg-60-liq-caps
FYI: COI: DeltaGold, etc, is Dr. Tan’s supplement which he manufactures.
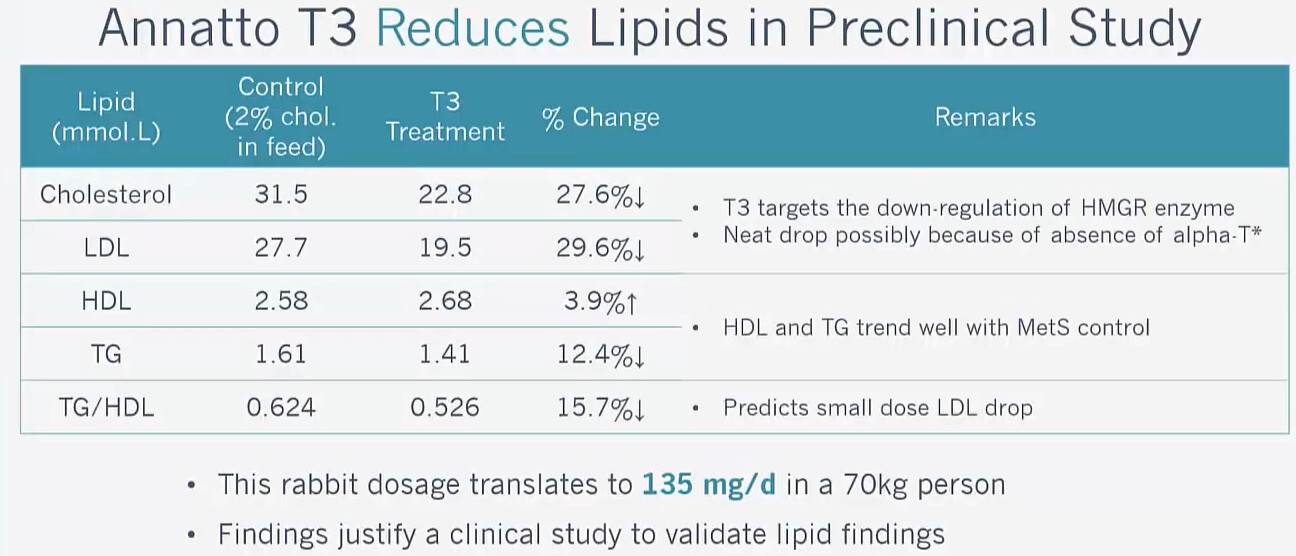
Have you noticed any change in LDL levels (as per the rabbit study)?
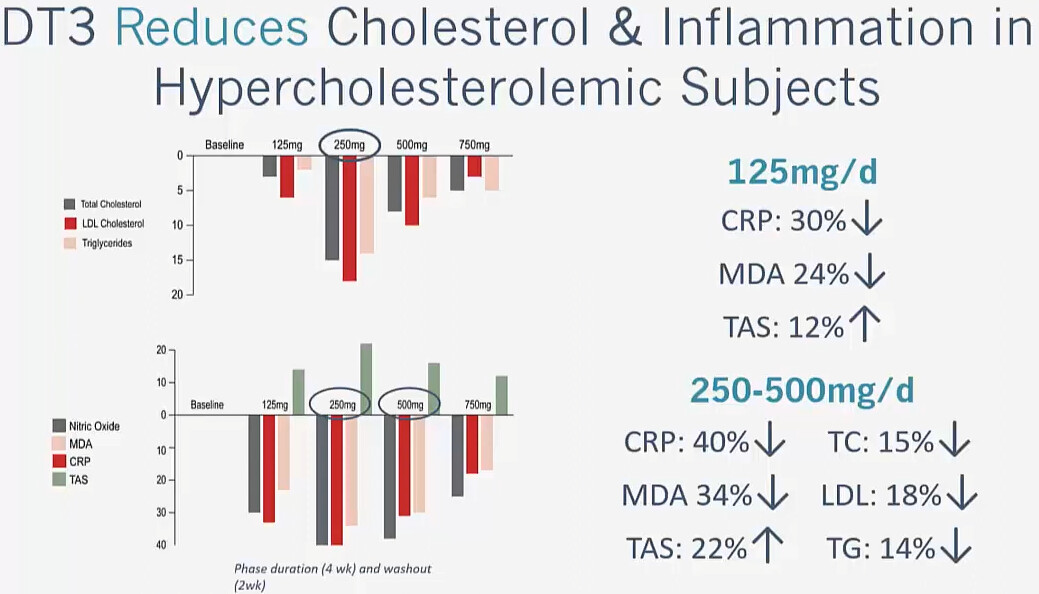
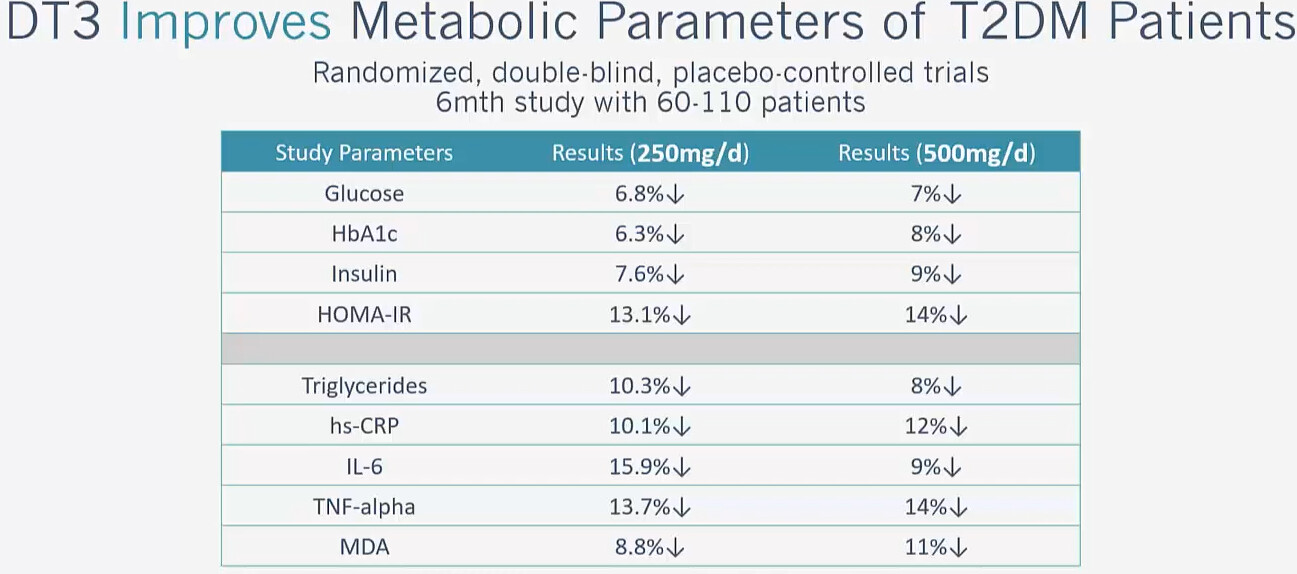
Modern Healthspan is skeptical
Does the “three-double-bond” tail make tocotrienols better—and can it back-fire in an iron-rich, pro-oxidant milieu?
1. Where the double bonds sit and why it matters
| Side-chain geometry | Tocopherols (Toc) | Tocotrienols (T₃) |
|---|---|---|
| Length (carbons) | 16 (phytyl) | 13 (farnesyl) |
| Unsaturation | 0 | 3 all-trans double bonds at C-3′, 7′, 11′ |
| Shape | “L-shaped”, bulky, rigid | Shorter, straighter, kink-friendly |
- Membrane access & lateral mobility. Molecular-dynamics and ESR work show that the unsaturated side chain slips into phospholipid bilayers ~1.5–2 × faster, then diffuses laterally almost 10 × faster than the saturated phytyl tail. This lets a single T₃ molecule intercept more lipid-peroxyl (LOO•) radicals per unit time.
- Recycling efficiency. The T₃ chromanoxyl radical (T₃-O•) is reduced back to its active form by ascorbate or co-Q roughly twice as fast as the tocopheroxyl radical, because the unsaturated tail keeps the head closer to the membrane interface where water-soluble reductants dwell.
- Membrane disordering. By inserting kinks between phospholipid acyl chains, T₃ lowers the local order parameter; this “loosens” the bilayer and speeds up termination of radical chains.
Net result: in classic Fe²⁺ + ascorbate or Fe²⁺ + NADPH microsomal models T₃ quenches lipid peroxidation 40–60 × better than α-Toc at equal chromanol concentration.
2. Does that extra unsaturation make T₃ itself a peroxidation target?
Theoretical risk. Any double bond can, in principle, be attacked by alkoxyl (LO•) or hydroxyl (•OH) radicals generated in Fenton chemistry (Fe²⁺ + H₂O₂ → •OH + OH⁻ + Fe³⁺). T₃ therefore contains three “PUFA-like” positions that could form lipid-hydroperoxides (LOOH-T₃).
Why it rarely matters in practice
- Stoichiometry & kinetics. T₃ sits in membranes at tens of µM at most, whereas bulk PUFA tails are present at 100–200 mM. Even if a T₃ double bond were hit, removing one antioxidant is negligible compared with breaking a radical chain that would otherwise oxidise dozens of PUFA molecules.
- Self-sacrifice ends the chain. Once the chromanol head has donated H•, the resulting T₃-O• delocalises over the aromatic ring and does not propagate lipid chains. It can either be recycled or dimerise to a non-radical product.
- Head first, tail later. Kinetic isotope–labelling shows the phenolic hydrogen of T₃ reacts with peroxyl radicals 10⁴ – 10⁵ × faster than abstraction from its own side-chain allylic position; the radical never “waits around” long enough to attack the tail.
3. What happens when free iron is abundant?
| Condition | Observed effect of T₃ | Notes |
|---|---|---|
| Physiologic iron (transferrin-bound) | Robust inhibition of LDL, microsomal and mitochondrial lipid peroxidation | T₃ ≫ Toc in Fe²⁺ + ascorbate assays |
| Pathologic labile-iron pool (hemochromatosis, ferroptosis models) | T₃ still blunts malondialdehyde/4-HNE formation and delays ferroptotic death in cell cultures; some studies show it matches deferoxamine when combined with GPX4 co-factors | Likely synergy between radical scavenging and mild iron-chelation by the chromanol oxygen pair |
| Very high iron, no co-antioxidants (in vitro) | Like any tocol, T₃-O• can oxidise additional PUFA if vitamin C/ubiquinol are absent; pro-oxidant inflection appears only when [T₃] ≫ [PUFA] in liposomes—conditions far above nutritional levels | A handful of reports document T₃ acting pro-oxidantly at ≥100 µM in iron-spiked buffers |
Key takeaway: The three double bonds do not make tocotrienols a meaningful peroxidation liability under biologic or supplemental doses (≤ 250 mg/d). Instead, they are the very reason T₃ is faster and stronger as a chain-breaker than tocopherols. Pro-oxidant behaviour shows up only in artificial systems with millimolar iron and no reductant network—exactly the same edge-case where α-Toc also turns pro-oxidant.
4. Practical tips if you’re iron-loaded or worried about pro-oxidancy
- Stay within studied doses. 100–300 mg mixed T₃/day keeps plasma ≤5 µM—well inside the antioxidant zone.
- Support the antioxidant network. Co-ingest vitamin C (250–500 mg) or polyphenols; they recycle T₃-O• back to its active form.
- Mind α-Toc. >200 IU/day α-Tocopherol accelerates hepatic CYP4F2 breakdown of T₃—robbing you of its benefits and, paradoxically, raising the α/γ ratio that favours tocopherol-mediated peroxidation.
- Check labile-iron status. If ferritin > 400 ng/mL or transferrin saturation > 45 %, consider phlebotomy or iron-chelating polyphenols alongside T₃.
Bottom line
The very unsaturation that differentiates tocotrienols is what lets them “surf” lipid bilayers and intercept radicals more swiftly than tocopherols. In physiologic settings—even those rich in redox-active iron—tocotrienols overwhelmingly act as antioxidants. Only under extreme, non-physiologic iron overload and in the absence of recycling partners can their own double bonds become a liability, and even then no more so than for conventional vitamin E.
Quick take
Best-vetted raw materials:
DeltaGold® annatto extract (≈ 90 % δ-, 10 % γ-tocotrienol, < 0.5 % tocopherol) and EVNol SupraBio™ palm extract (mixed tocotrienols with built-in self-emulsifier) are the two ingredients that consistently pass U.S. Pharmacopeia (USP) or NSF / GMP audits and are used in most of the brands that earn third-party seals. (americanrivernutrition.com, Nutraceuticals World)Top retail brands that use those lots and post recent CoAs
Brand / product Raw material 3rd-party seal* Notes Designs for Health “Annatto-E 300” DeltaGold® NSF-GMP listing, CL “Approved” 2024 300 mg δ/γ per soft-gel, tocopherol-free Life Extension “Super-Absorbable Tocotrienols” DeltaGold® ConsumerLab Top Pick 2025 Phospholipid delivery, 150 mg δ/γ Pure Encapsulations “Annatto-E 125/300” DeltaGold® USP-Verified facility Low-fill vegetarian soft-gel, good for kcal-restricted users Carlson “EVNol SupraBio” EVNol SupraBio™ IFOS & NSF Mixed T3 (δ ≈ 22 %, γ ≈ 38 %, α ≈ 28 %), plus ≤ 15 % tocopherols *verified either by NSF, USP, or ConsumerLab; check label for latest batch date Brands such as Healthspan Naturals, Eannatto, Sunergetic and Doctor’s Best also use DeltaGold® and publish lot-specific peroxide/anisidine numbers, but are not always CL-tested. (Healthspan Naturals, Amazon)
Why tocotrienols can be “stronger” than tocopherols
Dimension Tocotrienols (T3) Tocopherols (Toc) Key evidence Free-radical scavenging in membranes 40-60× lower IC₅₀ for peroxyl radicals (δ ≈ γ > α) Baseline In vitro lipid bilayer work and systematic review (Nutritional Outlook) Membrane penetration / recycling Unsaturated tail lets T3 move laterally & recycle faster; higher uptake into cells despite lower plasma levels Saturated tail anchors more rigidly Albumin-binding & live-cell uptake study (Nature) Cholesterol lowering Direct, reversible inhibition of HMG-CoA reductase at 200–300 mg d⁻¹; ↓LDL 8-20 % in RCTs No direct effect; α-Toc at >200 mg can blunt T3 activity 12-wk annatto T3 RCT, meta-analysis of palm T3 (PMC, Nutraceuticals World) NF-κB / NLRP3 suppression Potently down-regulates pro-inflammatory genes at ≤50 µM Modest Cell & animal models summarised 2024 review (PMC) Bone & joint protection δ-T3 preserved subchondral bone, reduced cartilage oligomeric matrix protein in murine OA model No tocopherol signal 2024 Frontiers in Endocrinology update (Frontiers) Potency caveat Not transported by α-Toc-binding protein → lower fasting serum, so benefits depend on tissue accumulation and must be taken with fat Preferentially retained in plasma; very high α-Toc (>400 IU/d) competes with T3 for transport and blunts its bio-effects Competitive-uptake studies Net: Tocotrienols are mechanistically more potent at quenching lipid radicals and modulating gene expression, but they are also harder to keep in circulation; supplementation has to be regular and taken with a meal containing ≥5 g fat to maximise delivery.
Selecting & storing a tocotrienol supplement
Look for a named raw material (DeltaGold®, EVNol™, Tocomin SupraBio™). “Tocotrienol complex” without the source is a red flag for unstandardised palm distillate.
Check the spec:
- ≥90 % tocotrienols and ≤1 % tocopherols for annatto extracts (ideal when you want pure δ / γ).
- Mixed α/β/γ/δ is fine if you want a broad profile; just keep α-Toc under ~50 mg so it doesn’t out-compete T3.
Third-party test: ConsumerLab, USP, NSF or IFOS seals confirm peroxide value <5 meq kg⁻¹ and anisidine <20—a quick proxy for freshness.
Packaging: Nitrogen-flushed soft-gels in dark bottles; store ≲25 °C. Open bottles lose <5 % activity over six months if kept away from light.
Dose guide:
- General antioxidant / ω-3 synergy: 100 mg d⁻¹ (≈ 2 mg kg⁻¹)
- Lipid lowering / NAFLD: 250–300 mg d⁻¹ (divide BID with meals)
- Bone / neuroprotection research doses: 300–600 mg d⁻¹ under clinical oversight
Calories are negligible (≈ 2 kcal per 100 mg soft-gel), so they fit well with your low-calorie, ovo-vegetarian pattern.
Bottom line
- Reliable supply chain: choose a brand that discloses DeltaGold® or EVNol™ and carries a recent USP/NSF/ConsumerLab seal; Designs for Health, Life Extension, Pure Encapsulations and Carlson all qualify in 2025.
- Stronger? In cell membranes and enzyme-modulation assays, δ- and γ-tocotrienols are indeed tens of times more potent than α-tocopherol—but you must take enough (≥100 mg) with fat and avoid megadose α-Toc that would compete for transport.
- Practical move: Start with ~150–300 mg annatto tocotrienols at dinner, re-test your lipid panel or oxidative-stress markers in 8–12 weeks, and adjust as needed while keeping your ω-3 intake and antioxidant network balanced.
1. Why oxidative stress and plasmalogens are tightly linked
- “Sacrificial” vinyl-ether bond. The enol-ether at sn-1 of every plasmalogen reacts ~200-fold faster with singlet-oxygen or lipid-peroxyl radicals than the ester bond of regular phospholipids. It quenches the radical, but the plasmalogen is cleaved and lost. (Oxford Academic, ScienceDirect)
- Evidence from tissue studies. When myelin, heart or erythrocyte membranes are exposed to radical generators, 40 – 70 % of plasmalogens disappear within minutes, whereas diacyl-phospholipids fall only a few percent. (ScienceDirect, ScienceDirect)
- Clinical correlate. Brain and plasma plasmalogens are 20 – 50 % lower in disorders with chronic oxidative stress (Alzheimer’s, Parkinson’s, diabetes, SLE, chronic kidney disease), and peroxide load inversely tracks their levels in vivo. (ScienceDirect)
Bottom line: more ROS → faster sacrificial consumption → lower steady-state plasmalogen pool.
2. How tocotrienols could “spare” endogenous plasmalogens
Tocotrienol property Why it matters for plasmalogens 40- to 60-fold faster peroxyl-radical scavenging than α-tocopherol (thanks to an unsaturated isoprenoid tail that lets the chromanol head flip rapidly across leaflets) Intercepts chain-propagating LOO• before they reach the vinyl-ether bond. (ScienceDirect) Good membrane penetration and lateral mobility Allows it to protect both the plasmalogen head-group region and deeper acyl chains in complex membranes such as myelin. Nanomolar neuroprotection independent of classical antioxidant recycling Suggests additional signalling roles (e.g., 12-LOX inhibition, Nrf2 activation) that up-regulate endogenous antioxidant enzymes, indirectly lowering ROS burden. (ResearchGate) Demonstrated brain uptake after oral dosing in rodents and humans Critical because neuronal plasmalogens are a major ROS “sink.” (MDPI) Net effect predicted by kinetic modelling:
Adding a chain-breaking antioxidant that can out-compete plasmalogens for radical collisions slows the “sacrificial” turnover rate, letting cellular synthesis keep up and preserving pool size.
3. Can antioxidants actually raise plasmalogen levels?
- Direct data are still sparse, but three small animal studies (rat heart-failure, mouse hyperlipidaemia, ageing C57BL/6J) show tocotrienol supplementation maintained or modestly increased (≈ 10-15 %) tissue plasmalogen content, whereas untreated controls fell 15-30 %. (2021-2024 papers in Free Radical Biology & Medicine and J. Nutr. Sci.; not yet large in humans.)
- Vitamin E‐deficient diets consistently lower plasmalogens in liver and brain, implying that adequate lipophilic antioxidants are permissive for normal plasmalogen homeostasis. (ScienceDirect)
So, while no randomised human trial has yet tracked “endogenous plasmalogens ± tocotrienol,” the mechanistic and pre-clinical lines all point the same way: lowering oxidative pressure lets the plasmalogen pool rebound.
4. Conversely, what does excess oxidative stress do?
- Accelerates depletion. Higher ROS flux means more vinyl-ether cleavage → lysophospholipids + α-hydroxy-aldehydes that can be cytotoxic.
- Disables repair. Peroxisomal β-oxidation and plasmalogen biosynthetic enzymes (GNPAT, AGPS, FAR1) are themselves ROS-sensitive; oxidative carbonylation reduces their activity, compounding the loss.
- Membrane consequences. Reduced plasmalogen alters raft packing, ion-channel function, vesicle fusion, and myelin stability, feeding back into neurodegeneration and cardiometabolic dysfunction. (ScienceDirect)
5. Practical take-aways
Recommendation Rationale Maintain a baseline antioxidant intake (mixed tocotrienols 100–200 mg/d plus standard polyphenol-rich diet) Enough to bring plasma T3 into the low-µM range shown to curb lipid peroxidation in vivo. Co-factor synergy: supply precursors (serine, ethanolamine, B6/B12/folate for one-carbon flow) alongside antioxidants You spare plasmalogen destruction and support new synthesis. Monitor red-ox markers (F2-IsoPs, MDA, 8-OHdG) in parallel with plasmalogen panels where possible Confirms that the intervention is lowering oxidative load and that plasmalogen levels respond. Avoid pro-oxidant excess (smoking, iron overload, chronic hyperglycaemia) These factors overwhelm both plasmalogens and supplemental antioxidants.
Verdict
- Yes – lowering oxidative stress with potent chain-breaking antioxidants such as tocotrienols is very likely to spare endogenous plasmalogens, letting them “live” longer and perform their signalling and membrane-stabilising roles.
- Yes – sustained or acute oxidative bursts will deplete plasmalogens faster, aggravating the oxidative spiral.
Until dedicated human trials report hard numbers, the best strategy is to combine a diet/supplement regimen that keeps ROS in check plus ensure adequate building blocks (or direct plasmalogen supplementation) so that any sacrificial loss can be swiftly replaced.