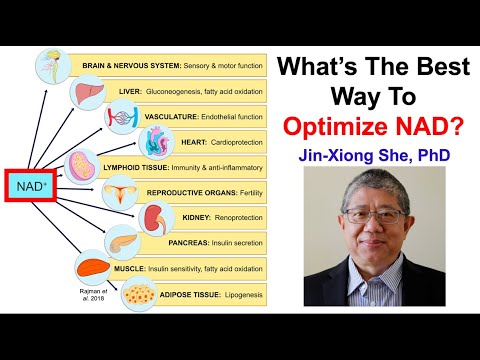
Personally I am not of the view that NAD supplementation is anything really significant, but this was released in June 2025.
https://www.nature.com/articles/s44324-025-00067-0
Abstract
Nicotinamide adenine dinucleotide (NAD+) is a coenzyme involved in a plethora of physiological reactions, with a key relevance in supporting mitochondrial function. Due to its critical role in these cellular processes, declining levels of NAD+ are associated with general aging and chronic disorders, including cognitive decline, sarcopenia, and metabolic diseases. These conditions are also typified by loss of mitochondrial health through dysfunction of homeostatic components such as mitophagy, unfolded protein response, and the antioxidant system. Therefore, raising cellular NAD+ through vitamin B3 family precursors or via drug-based interventions has become a broadly used strategy to restore mitochondrial and organismal homeostasis, with NAD+ precursors becoming a popular supplementation approach. As increasing components of the NAD+ biology are unraveled, this comprehensive review summarizes the advances in mechanisms of NAD+ metabolism and its modulation via compound-based strategies. Furthermore, it highlights the role of NAD+ in mitochondrial homeostasis in aging and disease conditions, the latest results of NAD±boosting therapeutics in clinical trials, and areas of further translational development.
O3:
At‑a‑glance summary
| Section of the review | Key points |
|---|---|
| NAD⁺ homeostasis | Walks through the four canonical biosynthetic routes (de novo, Preiss–Handler, classical salvage, NRK‐mediated salvage) and the “reduced” NRH/NMNH path discovered in 2023–24. Highlights tissue‑specific enzyme expression and newly mapped fluxes between liver, blood, and peripheral organs. (Nature) |
| Sub‑cellular transport & compartmentalisation | Details the 2021 discovery of the inner‑mitochondrial carrier SLC25A51 and proposes Slc25a45 as an NMN shuttle; discusses connexin‑43 pore for extracellular NAD⁺ and ENT/SLC29A family for NR. (Nature) |
| Mitochondrial regulation | Explains how high NAD⁺/NADH ratios fuel the TCA/ETC and how sirtuins (SIRT1/2/3/7) orchestrate mitophagy, UPR^mt, fusion–fission balance and antioxidant defences. (Nature) |
| Interventions | Reviews evidence for direct IV NAD⁺, vitamin‑B₃ precursors (NAM, NR, NMN, NRH), CD38 or PARP inhibition, and emerging enzyme‑activating drugs ; summarises 2022–25 clinical trials in cognition, sarcopenia, fatty‑liver and heart failure. (Nature) |
| Microbiome axis | Surveys rodent data showing NR‑driven shifts in gut taxa and bile‑acid signalling, but notes minimal effects in the first human pilot. (Nature) |
| System‑level model (Fig 3) | Presents a new organ‑flux diagram integrating tracer studies that chart precursor conversion (e.g. oral NR → hepatic NAM → systemic salvage). (Nature) |
What is novel about this review?
- Unified flux map. Figure 3 stitches together disparate isotope‑tracer studies into the first holistic, organ‑by‑organ traffic diagram of NAD⁺ metabolites, something earlier reviews only described qualitatively. (Nature)
- Transporter update. It is the first review to incorporate SLC25A51 (mitochondrial NAD⁺ importer) and the tentative Slc25a45 NMN carrier, shifting the field’s view from “NAD⁺ can’t cross the inner membrane” to an import‑competent model. (Nature)
- Reduced precursors. Summarises 2023–24 work on NRH/NMNH as potent, CD38‑resistant boosters that feed through a distinct NADH intermediate. (Nature)
- Microbiome and eNAMPT. Brings gut‑microbe utilisation of host precursors and exosome‑borne NAMPT into the mainstream mitochondrial discussion. (Nature)
- Clinical horizon scan. Tables the very latest (2024–25) Phase II data, including IV‑NAD⁺ boutique‑clinic protocols, which previous syntheses had not covered. (Nature)
Collectively, the paper serves as a 2025 state‑of‑the‑field handbook, knitting molecular mechanisms to translational endpoints.
Critique
Strengths
- Breadth with mechanistic depth. Unlike earlier high‑level narratives, the authors drill into enzyme kinetics, transporter topology and redox shuttles while still covering therapeutics—a valuable one‑stop reference.
- Currency. Citations run through March 2025 and include pre‑prints now in press, giving readers the freshest snapshot available.
- Didactic figures. The pathway (Fig 1) and flux (Fig 3) diagrams are clear and already cropping up in conference slide decks.
Limitations & missed opportunities
| Issue | Why it matters |
|---|---|
| Safety discussion is thin. Potential oncogenic risks of chronic NR (e.g. breast‑cancer metastasis signal) are relegated to a short paragraph and no risk–benefit framework is offered. (Pharmacy Times) | |
| Hype vs evidence. The review notes but doesn’t dissect the commercial boom in IV NAD⁺ and supplements; independent trials show small or null effects and media‑fuelled expectations remain unchecked. (New York Post) | |
| Conflicting clinical data. Null results (e.g. NR in metabolic syndrome) get scant attention, and heterogeneity in trial design (dose, route, biomarkers) is not critically appraised. | |
| Cancer & immunometabolism. Emerging work on NAD⁺ modulation in tumour micro‑environments and adaptive immunity is mentioned only in passing, despite clear therapeutic tensions. | |
| Long‑term pharmacology. The authors acknowledge knowledge gaps but offer little guidance on dosing ceilings, duration or interaction with polypharmacy—topics clinicians now face. (PMC) |
Overall verdict
This is an authoritative, up‑to‑date synthesis that will quickly become a citation staple. Its chief weakness is a relative lack of scepticism—particularly around safety signals and clinical efficacy—which readers must supplement with more critical literature. Incorporating a formal risk assessment and a structured comparison of trial outcomes would have strengthened the translational relevance.