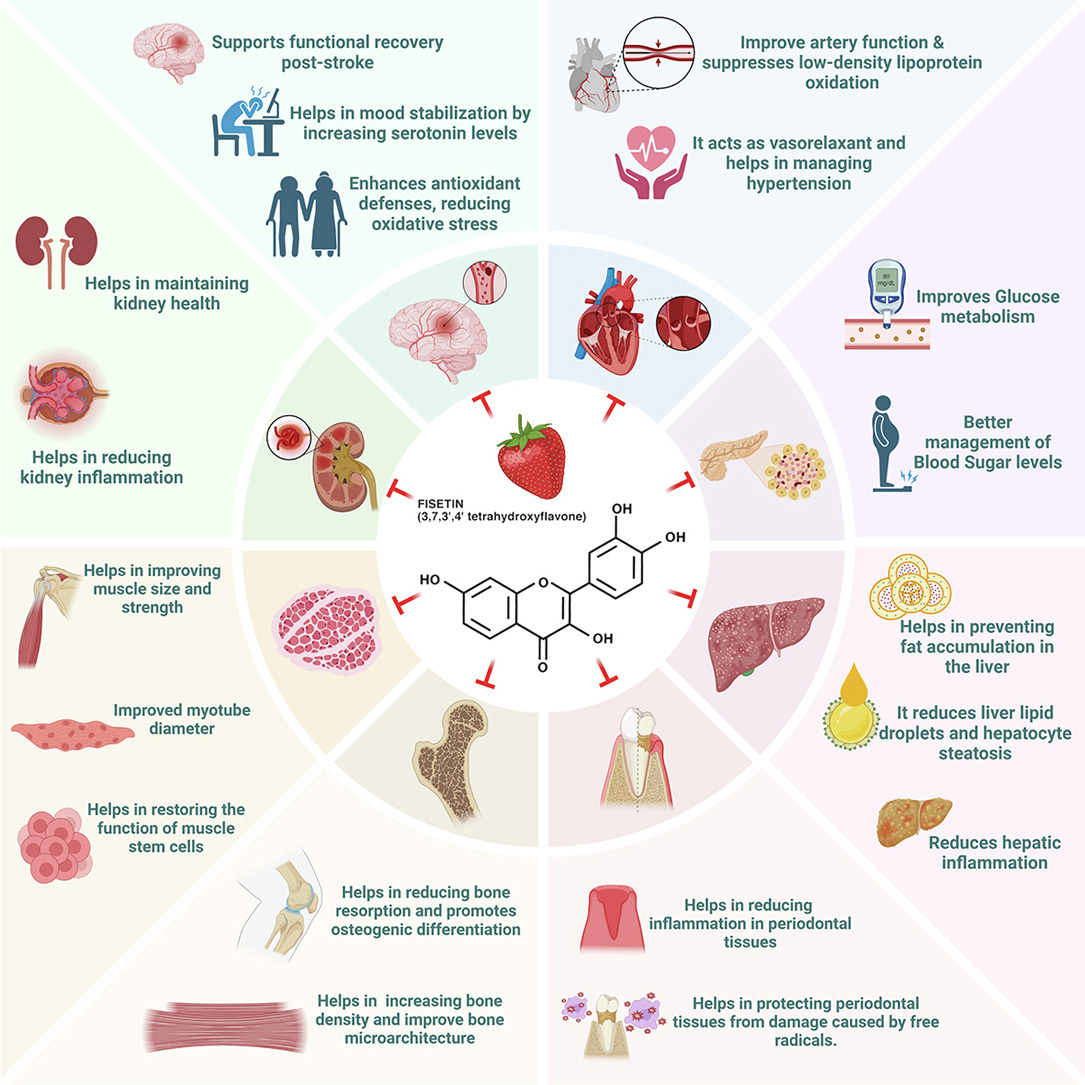
Fisetin as a Senotherapeutic Agent: Evidence and Perspectives for Age-Related Diseases
Highlights
-
Fisetin induced apoptosis in many, but not all, types of senescent cells in vitro
-
Fisetin can reduce senescent cell numbers and attenuate pathology in animal models
-
Ongoing clinical trials are evaluating the safety and efficacy of fisetin in humans
-
Reliable, sensitive, and accessible measures of efficacy remain to be validated
-
Fisetin’s low bioavailability and rapid metabolism may hinder clinical translation
Abstract
Fisetin, a flavonoid naturally occurring in plants, fruits, and vegetables, has recently gained attention for its potential role as a senotherapeutic agent for the treatment of age-related chronic diseases. Senotherapeutics target senescent cells, which accumulate with age and disease, in both circulating immune cell populations and solid organs and tissues. Senescent cells contribute to development of many chronic diseases, primarily by eliciting systemic chronic inflammation through their senescence-associated secretory phenotype. Here, we explore whether fisetin as a senotherapeutic can eliminate senescent cells, and thereby alleviate chronic diseases, by examining current evidence from in vitro studies and animal models that investigate fisetin’s impact on age-related diseases, as well as from phase I/II trials in various patient populations. We discuss the application of fisetin in humans, including challenges and future directions. Our review of available data suggests that targeting senescent cells with fisetin offers a promising strategy for managing multiple chronic diseases, potentially transforming future healthcare for older and multimorbid patients. However, further studies are needed to establish the safety, pharmacokinetics, and efficacy of fisetin as a senotherapeutic, identify relevant and reliable outcome measures in human trials, optimize dosing, and better understand the possible limitations of fisetin as a senotherapeutic agent.
…/…
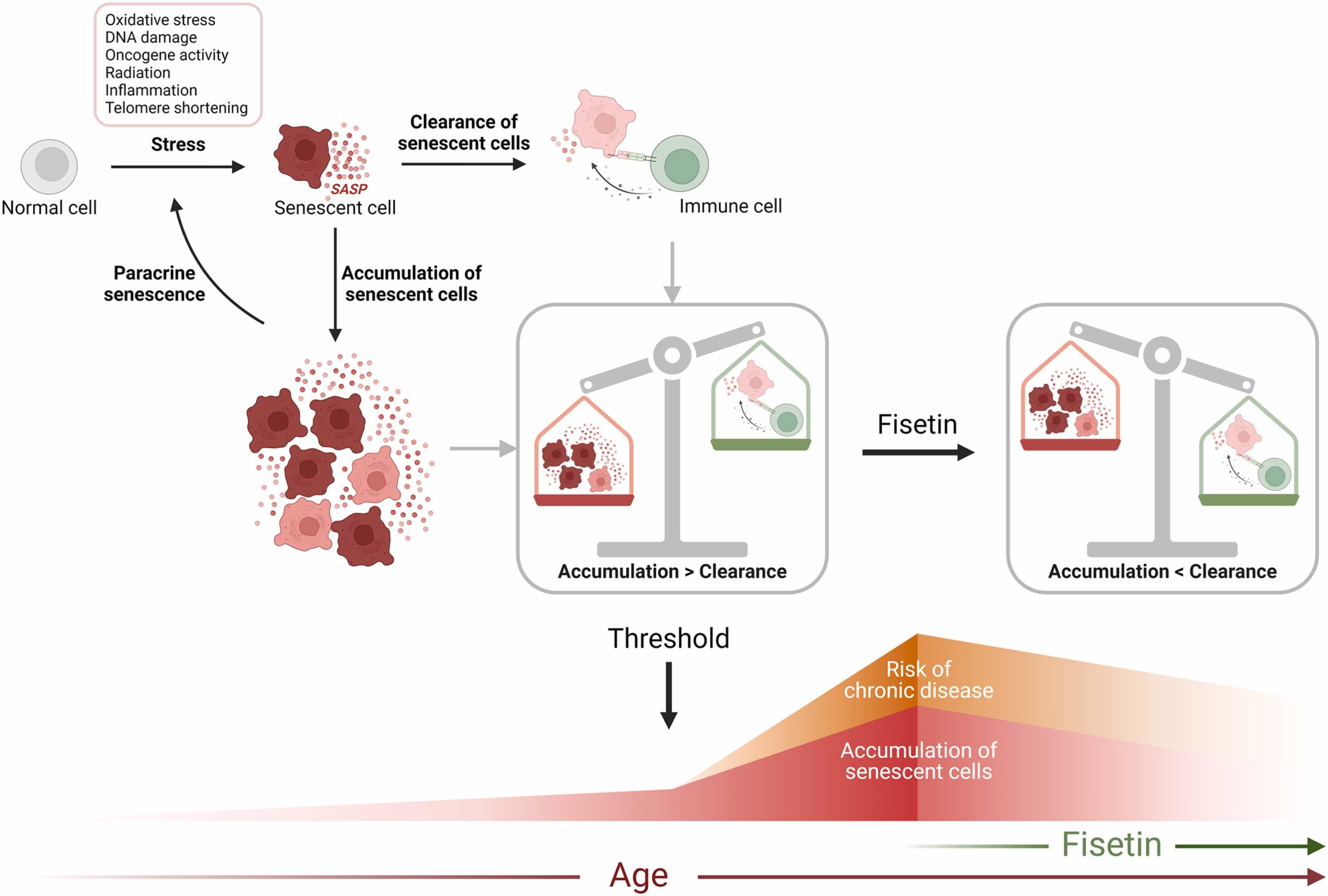
Fig. 1. Senescent cell accumulation and the threshold theory. Intra- and extracellular stressors can induce cells to enter a state of cellular senescence characterized, among others, by stable cell cycle arrest, resistance to apoptosis, increased generation of reactive oxygen species, and the acquisition of a senescence-associated secretory phenotype (SASP) (Wang et al., 2024). The SASP can in turn induce senescence locally in neighboring cells, through the “bystander effect”, but also in distant cells systemically (da Silva et al., 2019). Senescent cells are generally subjected to immunosurveillance. However, when the rate of generation of new senescent cells reaches a threshold that exceeds the rate of clearance by immune cells, in part due to declining immune function, senescent cells accumulate in tissues. This contributes to organ and tissue dysfunction, potentially leading to the development of chronic diseases (Chaib et al., 2022; Childs et al., 2017; Kirkland and Tchkonia, 2020; Ovadya et al., 2018; Tasdemir et al., 2016). Fisetin, as a potential senotherapeutic, could reduce senescent cell burden to a level where the immune system can again effectively control and prevent the accumulation of senescent cells, and thereby delay the onset and progression of chronic disease. Figure created with BioRender.
https://www.sciencedirect.com/science/article/pii/S0047637424000952
As far as I am concerned, fisetin is a chronic ‘promising low hanging fruit’, 100% available - even in a liposomal way - for everyone everywhere, and pretty cheap. Being so easy to get, it is taking too long - IMHO - for researchers to bring it to the antiaging’s first line definitely. BTW It has also shown real benefits in clearing senescence on old sheep’s tissues and organs, as the following study reveals.