The cost would be lower than a full body MRI since you’re only scanning a specific part and for shorter.
On this note, because dutasteride can make it harder to find prostate cancer, my husband is now getting a yearly MRI at Stanford to monitor.
Expanding the List of Adverse Experiences
I interviewed 29 formerly incarcerated youth offenders about their childhoods for my recently published book, Before Their Crimes: What We’re Misunderstanding About Childhood Trauma, Youth Crime, and the Path to Healing. I asked many questions about home life, parents, school, and never asked specifically about problematic experiences, yet the open-ended recounting of childhood memories revealed the ACE scores of the people I spoke with as clearly as if they had checked the boxes on an ACE screen.
Their descriptions of childhood convinced me that the ten ACEs did not capture the difficulties that impinged powerfully on their developing selves. The memories they shared led me to create another list, first among which was the death of a parent. In my small sample, nearly 30% had lost a parent when they were under the age of 15, and in every case, a cascade of other risks and ACEs followed.
I added ten more, each of which can and often does put children at increased risk of negative outcomes. I included having parents who were teenagers themselves; experiencing foster care; multiple home moves; school moves during elementary, middle, and high school; being bullied; school suspensions and expulsions; having a relative who is a gang member; being introduced to crime by a family member; and witnessing gun violence. Even this list does not exhaust derailing experiences that can occur.
Read the Full story: The List of ACEs Should Be Longer | Psychology Today
References
Bay Area Research Consortium on Toxic Stress and Health (2018) Pediatric ACEs and Related Life Events Screener (PEARLS). https://globalprojects.ucsf.edu/project/bay-area-research-consortium-toxic-stress-and-health
Felitti, V., Anda, R., Nordenberg, D., Williamson, D., Spitz, A., Edwards, V., Koss, M., & Marks, J. (1998) Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The adverse childhood experiences (ACE) study. Amer J of Preventive Medicine, 14(4).
Childhood Stress Echoes: New Review Links Early Adversity to Accelerated Brain Aging and Neurodegenerative Risk
Date: December 3, 2025 Source: International Journal of Molecular Sciences
A comprehensive narrative review published in the International Journal of Molecular Sciences synthesizes evidence linking Adverse Childhood Experiences (ACEs) to the pathogenesis of neurodegenerative diseases (NDs) like Alzheimer’s (AD), Parkinson’s (PD), and Multiple Sclerosis (MS). The authors argue that “toxic stress” experienced early in life does not merely vanish but undergoes “biological embedding,” manifesting decades later as accelerated cognitive decline and structural brain atrophy.
The central thesis posits that chronic dysregulation of the Hypothalamic–Pituitary–Adrenal (HPA) axis serves as the primary transducer of psychosocial trauma into cellular pathology. This “allostatic load” disrupts glucocorticoid signaling, leading to a cascade of systemic inflammation and mitochondrial dysfunction. Specifically, the review highlights that persistent elevation of cortisol downregulates hippocampal glucocorticoid receptors (GR) via FKBP5 methylation, impairing feedback loops and leaving the brain vulnerable to excitotoxicity.
Crucially, the authors map these stress pathways to specific neurodegenerative mechanisms. Chronic activation of the NF-κB pathway drives the release of pro-inflammatory cytokines (IL-6, TNF-α), causing microglial priming—a state where brain immune cells overreact to future insults. This neuroinflammation exacerbates oxidative stress, evidenced by mitochondrial DNA (mtDNA) depletion and telomere shortening. Ultimately, this cellular exhaustion compromises proteostasis, facilitating the aggregation of misfolded proteins such as amyloid-beta, tau, and alpha-synuclein, the hallmarks of AD and PD. The paper proposes that monitoring stress-associated biomarkers could provide a “preventive window” decades before clinical symptoms arise.
Mechanistic Interpretation for Longevity
- HPA-Mitochondrial Axis: Chronic cortisol acts as a metabolic uncoupler. By altering FKBP5 expression, early stress induces glucocorticoid resistance, preventing the “off-switch” for inflammation. This forces mitochondria into a chronic high-output state, increasing Reactive Oxygen Species (ROS) generation and depleting NAD+, likely suppressing Sirtuin activity and AMPK signaling.
- Inflammaging & Autophagy: The study details how elevated IL-6 and TNF-α impair autophagy, the cellular cleanup process. This failure allows protein aggregates (tau, a-synuclein) to accumulate. The activation of cGAS-STING pathways by leaked mtDNA (from damaged mitochondria) is a probable downstream effector, further amplifying neuroinflammation.
- Vascular & Glial Involvement: Elevated GFAP (glial fibrillary acidic protein) levels highlight astrocyte reactivity, suggesting that the blood-brain barrier (BBB) is compromised early in this stress cascade, allowing systemic inflammatory factors to penetrate the brain parenchyma.
Novelty
The paper’s primary novelty lies in its temporal integration: it reframes neurodegeneration not as a disease of old age, but as a lifespan process initiated by pediatric “biological scars.” It specifically correlates non-biological triggers (ACEs) with specific molecular biomarkers (NfL, GFAP, FKBP5 methylation), validating the “biological embedding” theory with hard biochemical targets. It also formally introduces Benevolent Childhood Experiences (BCEs) as a quantifiable biological buffer that can neutralize these risks.
Actionable Insights for the “Biohacker Parent”
-
The “BCE” Protocol (Benevolent Childhood Experiences): The paper explicitly identifies positive experiences not merely as “nice to have,” but as biological counter-weights that can neutralize the effects of stress.
- Action: Audit your child’s BCE score just as you would their grades. Ensure the presence of at least 1–2 consistent, non-parental supportive adult figures (mentors/coaches) and predictable family routines (e.g., fixed dinner times).
- Mechanism: BCEs function as “epigenetic buffers,” preventing the methylation of FKBP5 and keeping the HPA axis responsive rather than reactive.
-
HPA Axis Calibration via Sleep:
- Action: Prioritize circadian entrainment over social/academic flexibility. Enforce darkness at night and morning sunlight exposure.
- Rationale: Disrupted sleep in childhood doesn’t just cause fatigue; it flattens the Cortisol Awakening Response (CAR). A robust CAR is essential for appropriate immune regulation and synaptic pruning.
-
Nutritional “Allostatic” Reduction:
- Action: Eliminate ultra-processed foods and added sugars to the greatest extent possible.
- Mechanism: The study highlights that oxidative stress and mitochondrial dysfunction drive neurodegeneration. A high-sugar diet creates “metabolic stress” that stacks with “psychological stress,” accelerating the threshold for mitochondrial exhaustion and microglial priming.
-
Distinguish “Positive” vs. “Toxic” Stress:
- Insight: Not all stress is damaging. “Positive stress” (e.g., a difficult exam, a sports match) with supportive buffering builds resilience. “Toxic stress” is adversity without support.
- Action: Do not aim to remove all stressors (snowplow parenting). Instead, focus on “scaffolding”—providing the emotional recovery period immediately after a stressor to ensure cortisol returns to baseline. This trains the GR feedback loop to shut off efficiently.
-
Environmental Toxin Audit:
- Action: Reduce exposure to endocrine disruptors (plastics/phthalates) and air pollution.
- Rationale: While the paper focuses on psychosocial stress, the biological pathway (oxidative stress/inflammation) is shared. Chemical stressors add to the “Allostatic Load,” making the child’s system more vulnerable to psychological insults.
Feasibility & Limitations for Parents:
- Biomarkers: It is generally not recommended to track serum biomarkers (NfL, IL-6) in healthy children due to invasiveness and lack of pediatric reference ranges.
- Observation over Data: Instead of blood tests, monitor behavioral biomarkers of HPA dysregulation: sleep regression, digestive issues (gut-brain axis), or persistent anxiety. These are the visible proxies for the internal cortisol state.
Study Parameters
- Institution: School of Medicine and Health Sciences, Tecnológico de Monterrey
- Country: Mexico
- Journal: International Journal of Molecular Sciences
- Rank: Q1 (Biochemistry & Molecular Biology); Impact Factor ~4.9–5.6
- Study Type: Narrative Review (Synthesis of existing in vivo and human cohort data)
Source paper (open access) From Early Adversity to Neurodegeneration: Stress Biomarkers as Predictive Signals for Lifespan Brain Health
As the researchers noted, exposure to microplastics has been linked to chronic health conditions, and previous research identified microplastic particles in human reproductive tissue.
In the abstract, the authors explained that parents’ exposure to environmental contaminants has been shown to “increase the risk of cardiometabolic disease” in their children, but parental exposure to microplastics as a distinct pollutant had “not been studied.”
Prenatal particulate air pollution exposure predicts arterial stiffness in childhood
Early-life environment is crucial for foetal programming and later-life development. Exposure to particulate air pollution during gestation may increase the risk of cardiovascular diseases (CVD) later in life. We investigated the association between exposure to PM2.5 (particulate matter with a diameter of 2.5 μm or less) during gestation and pulse wave velocity (PWV) in children.
Conclusion
PWV is an independent predictor of future CVD and all-cause mortality in the general population. Therefore, associations of air pollution exposure during gestation with childhood PWV highlight the potential long-term consequences on the child’s cardiovascular system from early life onwards.
https://academic.oup.com/eurjpc/advance-article/doi/10.1093/eurjpc/zwaf647/8382385?login=false
Microplastics Can Rewire Sperm, Triggering Diabetes in the Next Generation
Researchers at the University of California, Riverside have reported for the first time that a father’s exposure to microplastics (MPs) can lead to metabolic problems in his offspring. Using mouse models, the team uncovered a previously unrecognized way in which environmental pollution may influence the health of future generations.
Although MPs have already been identified in human reproductive tissues, this study, published in the Journal of the Endocrine Society, is the first to directly connect paternal exposure to MPs with long-term health effects in the next generation (the “F1 offspring”).
Linking paternal exposure to offspring health
MPs are extremely small plastic fragments, measuring less than 5 millimeters, that form as consumer products and industrial materials break down. Metabolic disorders describe a group of conditions that include elevated blood pressure, high blood sugar, and excess body fat, all of which raise the risk of heart disease and diabetes.
To uncover metabolic effects in F1 offspring, the researchers placed them on a high-fat diet. This strategy helps expose the impacts of paternal MP exposure that might otherwise be subtle under standard dietary conditions. The high-fat diet reflects common unhealthy eating patterns, such as the Western diet, and increases metabolic stress. Because the fathers consumed a normal diet, the obesity observed in the offspring was driven by diet rather than inherited eating behavior.
https://scitechdaily.com/microplastics-can-rewire-sperm-triggering-diabetes-in-the-next-generation/
Trauma or Toxic? A Deep Dive into the Impact of Stress on Kids’ Health
Most research on the health effects of stress focuses on adults, but a new review looks at how stress uniquely affects children.
In the most comprehensive review of its kind to date, UC San Francisco researchers found robust evidence that stress occurring as early as before birth or as late as adolescence can affect multiple conditions in kids, from asthma to mental health to cognitive functioning. The results appear Jan. 20 in the Annual Review of Psychology.
Among the most important findings:
- Stress can impact many areas at once — mental and physical health, learning and attention, behavior, and justice system involvement — though its effects are often studied in silos.
- Children exposed to similar stressors can experience different outcomes, influenced by factors like age, emotional regulation, caregiver-child relationships, and school and neighborhood quality.
- The health and well-being of caregivers can significantly influence how stress impacts a child.
- Interventions early in a child’s life can improve their immediate and long-term health, while lowering long-term health and social costs.
The review analyzed research from 153 sources spanning 75 years to distill key findings about the health effects of childhood adversity on children before they reach adulthood.
“Let’s not wait until adults have heart disease, cancer, or end up in jail or on the streets to ask whether early childhood stress impacted their outcome,” said the review’s first author, Nicki Bush, PhD, a UCSF professor of Psychiatry and Pediatrics. “We can see the impact of stress on children right now, and the evidence suggests that, for some, we should intervene immediately to prevent later disease.”
“For too long, research on childhood stress has viewed physical and mental health as siloed, but, if we want to make a meaningful difference in children’s lives, we need to rethink how stress impacts a child’s overall health,” said Bush. “Many of the same stress-induced biological processes that predict asthma and obesity are also associated with anxiety, ADHD, and worse academic performance.”
Full story:
https://www.ucsf.edu/news/2026/01/431356/trauma-or-toxic-deep-dive-impact-stress-kids-health
Full Research Paper:
Early Life Stress Effects on Children’s Biology, Behavior, and Health: Evidence, Mediators, Moderators, and Solutions
The Ghost in the Machine: How Early Life Stress “Programs” the Pediatric Epigenome and Lifelong Disease Risk
The prevailing narrative in longevity science often focuses on the “geroprotectors” we take in our 40s and 50s—Rapamycin, Metformin, or SGLT2 inhibitors. However, a comprehensive review published by the University of California, San Francisco (UCSF) in the Annual Review of Psychology (2026) suggests we are looking at the wrong end of the clock. The authors argue that the biological “calibration” of our aging rate begins not at mid-life, but in the womb and during early childhood Bush et al. (2026).
The “Big Idea” is Biological Embedding: the process by which environmental stressors—poverty, racism, and household dysfunction—“get under the skin” to alter gene expression and physiological set-points. This isn’t just psychological; it is a structural “wear and tear” known as Allostatic Load. The review synthesizes meta-analytic data showing that children exposed to high stress exhibit “weathered” biological systems, including flattened cortisol rhythms, elevated systemic inflammation (IL-6), and accelerated epigenetic aging—often before they reach puberty Evidence for biological embedding of adversity (2026).
Crucially, the paper identifies Sensitive Periods—windows of high plasticity where the brain and immune system are hyper-responsive to external cues. While this makes children vulnerable, it also provides a “Resilience Dividend.” Interventions that bolster Early Relational Health (the caregiver-child bond) have been shown to “recalibrate” the HPA axis and even slow epigenetic clocks at the cellular level. For the longevity community, this underscores a sobering truth: our maximum lifespan potential may be capped by “epigenetic scars” formed decades before we started our first longevity protocol Intervening after trauma (2024).
Impact Evaluation: The Annual Review of Psychology is a premier journal in the field. According to recent data, its Journal Impact Factor (JIF) is 24.8, evaluated against a typical high-end range of 0–60+ for top general science, therefore this is an Elite impact journal.
Part 2: The Biohacker Analysis
Study Design Specifications
- Type: Systematic and Meta-analytic Review (Synthesis of international human cohorts).
- Subjects: Human pediatric samples (Infants, Children, Adolescents). Major cohorts include ALSPAC (UK) and ECHO (USA).
- Lifespan Analysis: Not a direct mortality study; however, it utilizes Biological Aging proxies (Epigenetic Clocks, Telomere length) which are validated predictors of all-cause mortality.
- Control Groups: Varied across the 100+ cited studies; typically low-stress/high-SES counterparts.
Mechanistic Deep Dive
The paper analyzes aging through several priority pathways:
- HPA Axis & Cortisol: Chronic stress leads to Hypocortisolism (flattened diurnal slope). In longevity, this reflects a “burnout” of the adaptive stress response, linked to chronic systemic inflammation.
- Inflammatory Cascades: Stress triggers pro-inflammatory cytokines like IL-6 and CRP. This “Inflammaging” begins in childhood, potentially causing early vascular stiffening and metabolic dysfunction Chiang et al. (2022) meta-analysis.
- Epigenetic Clocks: Adversity is directly correlated with Epigenetic Age Acceleration (EAA). This suggests that the “DNA Methylation” aging program is accelerated by cortisol-mediated signaling at specific CpG sites Musci et al. (2023).
- Telomere Attrition: Significant correlation between early maltreatment and shortened telomeres, a hallmark of cellular senescence Coimbra et al. (2017).
Novelty
This paper moves beyond the “Adult Retrospective” model (asking 50-year-olds about their childhood) to Real-Time Biomarker Tracking in children. It identifies the Prenatal-to-3 window as the primary “programming” phase for the immune system and the Pubertal Transition as a secondary “recalibration” window where the HPA axis can potentially be “reset” Gunnar et al. (2019).
Part 3: Verified Claims
| Claim | Evidence Level | External Verification / Safety | Confidence |
|---|---|---|---|
| Early Life Stress (ELS) accelerates the Epigenetic Clock. | Level A | Colich et al. (2020) Meta-analysis: Biological aging and adversity | High [90%] |
| ELS causes “Blunted” Cortisol reactivity in adolescents. | Level A | Niu et al. (2012) ELS and HPA axis function into adolescence | High [85%] |
| Prenatal stress influences fetal DNA methylation. | Level B | Epigenome-wide meta-analysis of prenatal maternal stress (2023) | Medium [70%] |
| Child-Parent Psychotherapy (CPP) can “slow” the epigenetic clock. | Level B | Sullivan et al. (2024) CPP and lower pediatric epigenetic age acceleration | Medium [60%] |
| Neighborhood “Green Space” buffers family-level SES stress. | Level C | Roubinov et al. (2018) Role of advantageous neighborhood characteristics | Low [45%] |
Part 4: Actionable Intelligence
The Translational Protocol
The pediatric research highlights Early Life Stress (ELS) as a potent “gerontogen” that accelerates the biological clock before adulthood. For the Longevity Specialist, the clinical objective for adults with high Adverse Childhood Experience (ACE) scores is a strategy of Damage Control and Active Recalibration.
Getty Images
- The “Re-Calibration” Strategy: ELS programs a pro-inflammatory phenotype. Adults with ELS histories may require more aggressive anti-inflammatory protocols to maintain the same healthspan as low-stress peers. This includes higher dosages of Omega-3 fatty acids for systemic inflammation or the strategic use of Senolytics to address ELS-induced cellular senescence.
- Safety & Toxicity (Psychosocial Interventions): Unlike pharmacological compounds, interventions such as CPP and mindfulness have a high No Observed Adverse Effect Level (NOAEL). However, clinicians must be wary of “reliving” trauma without adequate support, which can cause acute cortisol spikes and exacerbate allostatic load.
- Human Equivalent Dose (HED): While behavioral interventions lack a chemical HED, systemic “cash transfers” act as biological modifiers. A monthly transfer of $1,000 has been linked to increased infant brain activity, suggesting that socioeconomic stability is a primary “dose” for preventing intergenerational stress-induced aging.
Biomarker Verification Panel
To track the progress of “recalibration” in adults with ELS histories, the following markers are critical:
-
Efficacy Markers: * Diurnal Cortisol Slope: Use 4-point saliva testing to detect “blunting,” a core sign of HPA axis dysregulation resulting from ELS.
- High-Sensitivity CRP (hs-CRP): Monitor to verify the reduction of chronic inflammation linked to childhood adversity.
-
Safety Monitoring:
- Cystatin C: Monitor kidney function, as Cystatin C provides a more sensitive assessment than creatininewhen implementing aggressive longevity protocols.
- ALT/AST: Essential for tracking liver health, especially since ELS history is correlated with altered metabolic flexibility and fatty liver risk later in life.
New Open Access Paper : Sugar rationing during the first 1000 days of life and lifelong risk of heart failure (Nature Communications)
The “1953 Protocol”—Early Life Sugar Restriction Delays Heart Failure by 2.6 Years
A new study leveraging a massive “natural experiment”—the end of World War II sugar rationing in the United Kingdom—reveals that restricting sugar intake during the first 1,000 days of life (conception to age two) significantly creates a “metabolic shield” against heart failure (HF) decades later. Researchers analyzed 61,193 participants from the UK Biobank, comparing those conceived during strict rationing (limiting adults to ~40g/day and children to near-zero) versus those born immediately after the restrictions were lifted in 1953, when sugar consumption instantly doubled.
The results are striking: individuals exposed to rationing in early life had a 14% lower lifetime risk of heart failure and, perhaps more critically for longevity enthusiasts, delayed the onset of the disease by approximately 2.6 years. This protective effect was dose-dependent; the longer the exposure to rationing (in utero plus infancy), the robust the protection. Crucially, this benefit was additive to genetic risk—meaning even those with high polygenic risk scores for heart failure saw risk reduction from early nutritional discipline. This suggests that the “sweet tooth” is not just a habit, but a biologically programmed vulnerability established before we can even speak.
Journal Context:
- Institution: Shantou University Medical College (China) / UK Biobank Data.
- Journal: Nature Communications (2026).
- Impact Evaluation: The impact score of this journal is ~14.7 (2024 JIF), evaluated against a typical high-end range of 0–60+ for top general science, therefore this is a High impact journal.
Biohacker Analysis: Technical Breakdown
Study Design Specifications
- Type: Human Observational Cohort (Natural Experiment / Regression Discontinuity Design).
-
Subjects: Humans (N = 61,193).
- Treatment Group: 38,737 (Exposed to rationing in utero and/or early infancy).
- Control Group: 22,456 (Born post-1953, unexposed).
- Dataset: UK Biobank (Age at recruitment: ~54 years).
“Lifespan” & Healthspan Analysis
- Mouse Lifespan Context: This is a human study. Unlike murine rapamycin studies Rapamycin extends life (2014)that show max lifespan extension, this study measures Healthspan Extension via delayed disease onset.
-
Effect Size:
- Delayed Onset: ~2.6 years (HF diagnosis occurred at age 63.2 vs. 60.6).
- Risk Reduction: ~14% lower hazard ratio (HR 0.87) for HF.
- Dose-Response: In utero exposure alone provided protection, but in utero + postnatal exposure (up to 2 years) yielded the strongest effect (HR 0.77).
Mechanistic Deep Dive
The authors propose the Developmental Origins of Health and Disease (DOHaD) hypothesis.
- Metabolic Programming: Excessive in utero glucose crosses the placenta, potentially altering fetal pancreatic beta-cell development and mitochondrial function in cardiomyocytes.
- “Sweetness” Calibration: Early exposure likely sets the “hedonic setpoint” for sugar. Unexposed infants may develop a lower threshold for satiety, reducing lifetime glycemic load.
- Pathway Implication: While not measured directly in this study, the phenotype suggests a lifelong reduction in insulin resistance and chronic inflammation (Inflammaging), key drivers of HFpEF (Heart Failure with preserved Ejection Fraction).
Critical Limitations
- Observational Nature: Despite the “natural experiment” design reducing confounding, it cannot fully rule out other post-war socioeconomic shifts (e.g., changes in antibiotic access or stress).
- Survival Bias: The cohort includes only those who survived to age ~54 to enroll in UK Biobank. The most susceptible individuals may have died earlier, potentially underestimating the benefit.
- “Sugar” Type: The study measures sucrose (cane/beet sugar). It does not directly account for modern High Fructose Corn Syrup (HFCS), though the biological impact of fructose load is likely comparable or worse.
Claim Verification & Hierarchy
Claim 1: Early-life sugar rationing reduces Heart Failure risk by ~14% in adulthood.
- Evidence Level C (Cohort/Natural Experiment): The study utilizes a regression discontinuity design, which is superior to standard observational studies but inferior to RCTs.
- Verification: Verified in source text (Source 728: HR 0.87).
- External Support: Supported by parallel analysis of the same dataset regarding diabetes and hypertension.
Claim 2: The protective effect is “additive” to genetic risk (Polygenic Risk Score).
- Evidence Level C: The study found no multiplicative interaction, meaning the diet worked regardless of genetic susceptibility.
- Verification: Source 739 states “A notable additive interaction was observed… individuals with high genetic susceptibility who were not exposed… exhibited an increased risk.”
- External Context: High genetic risk does not negate lifestyle interventions.
Claim 3: In utero exposure alone confers protection (~20% risk reduction for Hypertension/Diabetes in similar studies).
- Evidence Level C: The Science 2024 paper (Gracner) confirms in-utero effects. This paper (Tang) finds “Rationed in-utero only” HR = 0.79 (Source 1099).
- Translational Gap: The mechanism is inferred (fetal programming), not proven via biopsy/molecular analysis in this cohort.
- Safety Check: Reducing sugar in pregnancy is standard medical advice, but extreme carbohydrate restriction (ketogenic) during pregnancy lacks safety data.
Actionable Intelligence: The “1953 Standard” Protocol
Strategy: Mimic the scarcity of 1953 to induce the same metabolic resilience. This is a Prevention and Parentalprotocol, but likely benefits adults by resetting insulin sensitivity.
1. The Protocol (Dietary “Dose”)
-
Human Equivalent Dose (Dietary Limit):
- Pregnant/Nursing Mothers: < 40g added sugar/day.
- Children < 2 Years: 0g added sugar. (Strict adherence).
- Children > 2 Years: < 15g added sugar/day (approx. 3 teaspoons).
- Validation: These limits align with current WHO and AHA guidelines but are rarely followed.
2. Biomarker Verification Panel
To track if “sugar rationing” is working (for adults adopting this now):
- HbA1c: Target < 5.0% (indicates low average glucose).
- Fasting Insulin: Target < 5 uIU/mL (The gold standard for insulin sensitivity).
- Triglyceride/HDL Ratio: Target < 1.0 (High sugar intake drives Trigs up and HDL down).
- ApoB: Monitor for atherogenic particle number.
3. Feasibility & Cost-Benefit
- Cost: Negative. You save money by not buying processed sweets.
- Difficulty: High. Requires fighting the “bliss point” engineered into modern food.
- ROI: High. 14% risk reduction for HF is comparable to some pharmacological interventions (e.g., SGLT2 inhibitors) but without side effects or financial cost.
4. Safety & Contraindications
- Hypoglycemia: In non-diabetics, sugar restriction does not cause dangerous hypoglycemia due to gluconeogenesis.
- Eating Disorders: Strict elimination diets can trigger orthorexia. Psychological balance is required.
- Contraindication: None known for added sugar restriction. Essential carbohydrates can be obtained from vegetables/tubers.
Strategic FAQ: The Skeptic’s View
Q1: Is this effect actually just about calories, not sugar? A: The study attempted to control for this. Post-rationing, total caloric intake increased only slightly (~3%), but sugar intake doubled (Source 823). The specific harm of fructose (hepatic load, uric acid generation) is the likely culprit distinct from general caloric excess. Fructose and metabolic health (2018)
Q2: I was born after 1953. Is it too late for me? A: The developmental window is closed, but the mechanism (insulin resistance) is reversible. While you missed the “structural” benefits (e.g., optimal pancreatic beta-cell mass), adopting the “1953 Standard” now can still reduce metabolic burden. Reversal of type 2 diabetes (2019)
Q3: Does this finding apply to High Fructose Corn Syrup (HFCS)? A: Likely yes, and worse. The rationing involved sucrose (50% glucose/50% fructose). HFCS is often 55% fructose. Since fructose drives the hepatic de novo lipogenesis implicated here, modern sweeteners are likely more damaging than the 1950s sugar. Metabolic effects of fructose (2013)
Q4: Does this conflict with taking Metformin or SGLT2 Inhibitors? A: No, it is synergistic. SGLT2 inhibitors (e.g., Empagliflozin) lower heart failure risk by dumping glucose. Restricting sugar intake lowers the glucose load upstream. Combining them is a powerful “defense in depth” strategy. SGLT2 inhibitors for heart failure (2020)
Q5: Is there a “rebound effect” where restricted kids binge later? A: The data suggests the opposite. Early restriction appears to reset the “hedonic threshold,” leading to lower preference for sweets later in life. The “forbidden fruit” effect is psychological, but the metabolic programming is physiological. Early taste experiences and later food choices (2017)
Q6: Why focus on Heart Failure specifically? A: HF is often the terminal event of “Metabolic Syndrome” (Diabetes + Hypertension). By preventing the upstream drivers (as shown in the sister Science paper), you delay the mechanical failure of the heart pump.
Q7: How does this compare to Rapamycin? A: Rapamycin inhibits mTOR, which is activated by amino acids and insulin (sugar). Sugar restriction lowers insulin, indirectly reducing mTOR signaling. They target the same “nutrient sensing” axis.
Q8: Can pregnant women use artificial sweeteners instead? A: Caution advised. Some data suggests non-nutritive sweeteners may also alter the infant gut microbiome or metabolism. The safest “sweetener” for pregnancy is whole fruit.Artificial sweeteners and pregnancy outcomes (2016)
Q9: What is the “Number Needed to Treat” (NNT)? A: Based on the 4-5% population attributable fraction (Source 729), the NNT is high (dietary changes for many to save one case), but the cost is zero, making it a “public health best buy.”
Q10: Is there a genetic interaction? A: The study found an additive interaction (Source 739). If you have “bad genes” (High PRS), sugar restriction doesn’t fix the genes, but it removes the environmental trigger that pulls the trigger on the gun. You need the restriction more than someone with low genetic risk.
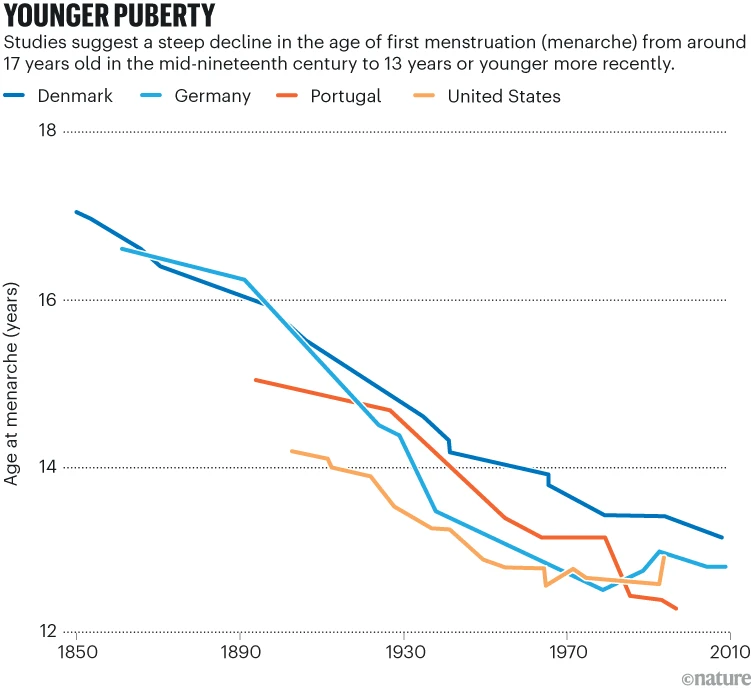
Girls are starting puberty younger — why, and what are the risks?
More girls are hitting puberty at eight or earlier. Researchers are exploring the causes, the consequences and what should be done.
Scientists have found a range of possible drivers for this change, with increasing body weight and obesity almost certainly playing a part. Some researchers suspect that exposure to hormone-disrupting chemicals or stress during childhood could be pushing puberty earlier, but studies have produced conflicting results. The trend has prompted the international organization the Endocrine Society to develop clinical-practice guidelines on puberty, to be published in mid-2026. The guidelines will reconsider how to treat girls on the border between typical and ‘precocious’ puberty, which has commonly been defined as before the age of eight in girls, but that some specialists argue should be younger.
Research over the past few years is also making the health risks of early puberty increasingly clear. Studies have linked it to greater risk of conditions including obesity, heart disease, breast cancer, depression and anxiety. Other research suggests that children who go through puberty earlier are more likely to experience discrimination because of their race or ethnicity, or otherwise be treated differently from their peers.
Read the full article: Girls are starting puberty younger — why, and what are the risks?
The Body Keeps Score: How Childhood Stress Rewires Everything
January 21, 2026
For decades, researchers studied stress as if it came in neat packages. Mental health specialists looked at anxiety and depression. Pediatricians examined asthma and infections. Neurologists tracked learning delays. Child development experts watched behavior problems. Nobody was connecting the dots. When Nicole Bush began her career as a pediatric psychiatrist at the University of California, San Francisco, she noticed something unsettling: the same children kept showing up in multiple clinical pictures. A kid struggling in school also had unexplained stomach problems and social difficulties. Another with anxiety also had poor immune function.
“For too long, research on childhood stress has viewed physical and mental health as siloed,” Bush says. “But if we want to make a meaningful difference in children’s lives, we need to rethink how stress impacts a child’s overall health.”
That rethinking just took a massive leap forward. In the most comprehensive review of its kind, Bush and her colleagues at UCSF analyzed 75 years of research across 153 studies to understand exactly how early life stress damages developing children. What they found wasn’t just shocking in scope. It was shocking in its systemic reach. Stress doesn’t just affect one system. It doesn’t just affect two. It affects virtually everything: mental health, physical health, learning, attention, behavior, even whether a child ends up involved with the justice system. And crucially, the same biological mechanisms that predict asthma and obesity are the very ones linked to anxiety, ADHD, and academic failure.
The implications are staggering. And they suggest we’ve been thinking about childhood trauma all wrong.
The Interconnected Storm
The old model was simple: adversity causes psychological damage. You process the emotional trauma and move forward. But what the UCSF review reveals is that stress doesn’t work like an emotional injury. It works like a biological cascade, rewriting the body’s fundamental operating systems.
When a child experiences prolonged stress (whether from abuse, neglect, poverty, discrimination, or loss), their stress response system doesn’t just activate once and settle. It stays activated. Their cortisol levels flatten into unhealthy patterns. Their autonomic nervous system remains in overdrive. Their inflammatory markers climb steadily upward. Their brain structures actually shrink in measurable ways, particularly in regions governing emotion regulation and decision-making.
Yeah, I can definitely believe the childhood stress thing. It’s funny how it manifests itself in different ways in adulthood. And the links to food are also very interesting, and it makes sense.