Results
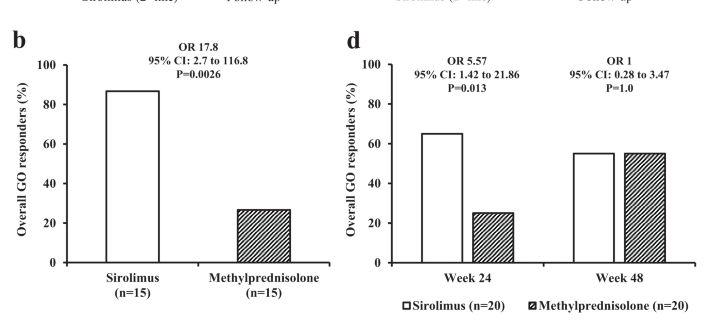
mTOR signaling pathway was found to be upregulated in patients with GO. In addition, studies in a mouse model of GO, in orbital fibroblasts and peripheral blood mononuclear cells (PBMCs) derived from GO patients showed a significant reduction in inflammatory mediators, adipogenesis and fibrosis after treatment with sirolimus. In 2007 and 2019 two cases of patients with GO unresponsive to glucocorticoids and successfully treated with sirolimus were described. Consistently with these results, two retrospective investigations showed that treatment with sirolimus, used at low dosage for 12 weeks, was followed by a greater overall response of GO compared with methylprednisolone at 24 weeks. In addition, a good response in diplopia and ocular motility restriction after treatment with sirolimus was reported by two case series. All of these studies reported a good tolerability of sirolimus. Finally, GO response to treatment was shown to correlate with the serum levels of sirolimus at the end of treatment.
Conclusions
Sirolimus may represent a cheap, effective, and safe alternative treatment for GO. In addition, serum levels of sirolimus may be used to predict the response to treatment. Randomized clinical trials are needed to confirm the efficacy of sirolimus on GO and establish the best possible treatment protocol.
The data reviewed so far suggest that sirolimus holds promise as a novel therapeutic option for patients with GO, especially in cases characterized by inflammatory and fibrotic features. Based on studies in vitro and in animal models, sirolimus may act in GO through multiple mechanisms involving mTORC1 inhibition: suppression of cytotoxic CD4+ T cell activity, downregulation of proinflammatory cytokines like IL-16, and reduction of fibroblast-mediated tissue remodeling via inhibition of the IGF-1 R–Src–mTOR axis. These combined effects could translate into both immunomodulatory and antifibrotic therapeutic benefits in GO.