Catching up on old posts. I do like that it lowers resting heart rate, so it might be better for peple who need to reduce that, but otherwise you make a convincing case that it’s not as good as the others mentioned.
BP = SV x HR x SVR
SV = Stroke volume
HR = Heart rate
SVR = Systemic vascular resistance
Beta blockers primarily act on BP via a reduced heart rate. Other anti hypertensive work on other parts of the equation. Amlodipine might increase a bit the HR so there might be a place for a beta blocker in addition to telmisartan, amlodipine and indapamide SR as an ultra low dose quadruple combination. Indeed, this paper published a few days ago makes the case for such combinations and notes the potential synergies between beta blockers and CCBs and ACEIs/ARBs: β-blockers are not all the same: pharmacologic similarities and differences, potential combinations and clinical implications 2024
However, the authors argue that bisoprolol is the best beta blocker based on clinical trials and pharmacokinetics. It seems that nebivolol hasn’t been studied enough to recommend it and suffers from great variability between individuals.
It turns out that in the two QUARTET trials they used bisoprolol (+ ARB + amlodipine + indapamide): Rilmenidine vs Telmisartan or other BP meds for Longevity - #63 by adssx
Is the response to antihypertensive drugs heterogeneous? Rationale for personalized approach 2024
The Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019, which estimated the proportion of mortality, years of life lost, and years of life lived with disability attributable to 87 behavioural, environmental, occupational, and metabolic risk factors, highlighted how the predominant share of deaths in the world is attributable to systolic BP values ≥ 110–115 mmHg with an estimate of 10.8 million avoidable deaths every year and 235 million years of life lost or lived with disability every year.
Furthermore, the combination of drugs that act on different mechanisms involved in the rise in BP values reduces the variability of the BP response to treatment and allows for a faster BP response to be obtained than that which can be obtained with increasing doses of a single drug and it is safe and well tolerated with a modest risk of hypotensive episodes, even when prescribed to patients with grade 1 hypertension.
The finding of a substantial heterogeneity in the individual response to the different treatments used in the PHYSYC study suggests the possibility of obtaining additional advantages with a personalization of the treatment, but in the current state of knowledge, this eventuality still appears rather theoretical, especially due to the difficulties in making personalized choices. Following the approach used in the PHYSIC study, one could hypothesize testing the patient’s individual response to a series of short periods of treatment before defining long-term therapy with one or more drugs, but this approach appears quite laborious.
Theoretically, it would be simpler to identify phenotypic characteristics that can predict a satisfactory response to a drug or a combination of drugs. Indeed, beyond the long-known modest sensitivity of black hypertensive patients to treatment with ACE-I, the limited availability to date of phenotypic indicators of response to a drug makes this approach difficult to pursue.
The progressive digitalization of healthcare systems is making enormous quantities of data available for machine learning systems which will allow the derivation of management algorithms for truly personalized antihypertensive therapy in the near future.
In the meantime, I guess the best approach is trial and error and/or a low-dose combination of the best-in-class agents (so telmisartan, amlodipine, indapamide SR, and bisoprolol?).
Provisionally accepted paper, we don’t have the full text yet: Association between types of antihypertensive medication and the risk of atrial fibrillation: A nationwide population study 2024
In monotherapy, ACEi and CCB had similar AF risks as ARB, while beta-blockers and diuretics showed higher AF risks than ARB. In combination therapy, ARBs/CCBs and ARBs/diuretics had the lowest AF risk, whereas ARBs/beta-blockers had the highest compared to ARB/CCB. Among the specific ARBs, the AF risk varied insignificantly, except for telmisartan and candesartan. In hypertensive patients receiving monotherapy, ACEi and CCB showed a similar AF risk as ARBs, while beta-blockers and diuretics were associated with a higher risk.Among those receiving combination therapy, ARBs/CCBs and ARBs/diuretics had the lowest AF risk, whereas ARBs/beta-blockers showed the highest risk. Various types of ARBs have different associations with AF risk.
So we’ve got nothing for now to tell us if they mean worse vs better?
I’ve been tapering off my nebivolol as I gradually increase telmisartan dose, so far BP is great (115/75) at 40mg tm, 2.5mg nb. Looks like I’m on the right track but that eventually adding in amlodipine might help further reduce future afib risk.
I tried to get dapagliflozin from India this time instead of empa (since I believe a study you posted a while ago showed decreased afib risk with dapagliflozin but not empa), but I prefer branded meds when ordering from India and was told that the manufacturer of Farxiga no longer allows sale in India😒
BTW I really appreciate the literature searches you do! I need to up my lit search skills.
Yes it’s a bit sad the summary doesn’t give that essential information ![]() We’ll see when we have the full text… But I’m not sure it’s a great paper tbh (given the journal + institutions).
We’ll see when we have the full text… But I’m not sure it’s a great paper tbh (given the journal + institutions).
Besides bisoprolol, carvedilol seems interesting, although it has a shorter half-life (6h) and therefore needs to be taken twice a day (vs once daily for bisoprolol). But it has interesting additional properties:
An Updated Prioritization of Geroscience- Guided FDA-Approved Drugs Repurposed to Target Aging 2024
One study found that the beta blocker carvedilol reduced oxidative stress-induced apoptosis in cardiomyocytes (see Supplement 1).
One meta-analysis of interventional trials reported carvedilol, a non-selective beta-blocker, to decrease mortality significantly more than beta-1 selective beta blockers in patients with either heart failure or myocardial infarction.
Today, four β-blockers are recommended for the treatment of HF (bisoprolol, carvedilol, metoprolol succinate (CR/XL) and nebivolol) in the European Guidelines.
Carvedilol-induced β1AR-Gi signaling also addresses the PI3K/Akt pathway and induces NOS3 activation, thereby promoting cardiomyocyte survival.
Carvedilol induces vasodilation via antagonism at peripheral α1AR inducing arterial vasodilation while concurrently suppressing reflex tachycardia via cardiac β1AR-blockade.
Carvedilol and its metabolites have anti-oxidative properties that manifest as antioxidation (e.g. reduced lipid peroxidation) and anti-inflammation. These capabilities are attributed to the chemical structure of carvedilol and its metabolites, which have a tricyclic carbazole structure.
Carvedilol – via the PKA/PGC1α pathway – leads to improved mitochondrial function in endothelial cells, resulting in protective/beneficial effects within the context of atherosclerosis.
Additionally, carvedilol - likely via modulation of potassium channels - stabilizes glucose homeostasis and attenuates hepatic glucose production, while enhancing muscular insulin signaling. Consistent with these observations, carvedilol has shown favorable in the context of metabolic disturbances in patients.
In Association of cardiovascular disease management drugs with Lewy body dementia: a case–control study 2023, the HR of carvedilol use with LBD was 0.67 [0.65-0.69], the best-performing beta-blocker and as good as telmisartan (but it’s an association study, not an RCT).
On the other hand, in the EPITERNA preprint, carvedilol was associated with a significantly shorter lifespan and was the worst-performing beta-blocker (again, association study).
In animal and computational models, it seems neuroprotective:
- Identification of Azelastine and Carvedilol as Cholinesterase Inhibitors via Structure-Based Virtual Screening of FDA-approved Drugs 2023
- Neuroprotective repositioning and anti-tau effect of carvedilol on rotenone induced neurotoxicity in rats: Insights from an insilico& in vivo anti-Parkinson’s disease study 2022
And there are three ongoing studies of carvedilol in NDDs:
- Cardiac Changes in Early Parkinson’s Disease: A Follow up Study (NCT04218968): “Twice daily oral doses of adrenergic blocker 12.5 mg or 25mg, according to patient tolerability.” => Ends in Dec 2025
- Adrenergic Blockers for Cardiac Changes in Early Parkinson’s Disease (Protocol 53136): “target dose will be 25mg twice daily” => Ends in Aug 2024
- Data Analysis for Drug Repurposing for Effective Alzheimer’s Medicines (DREAM)- Propranolol/Carvedilol Versus Atenolol/Bisoprolol/Sotalol (NCT05794997) => Ended in Dec 2023?
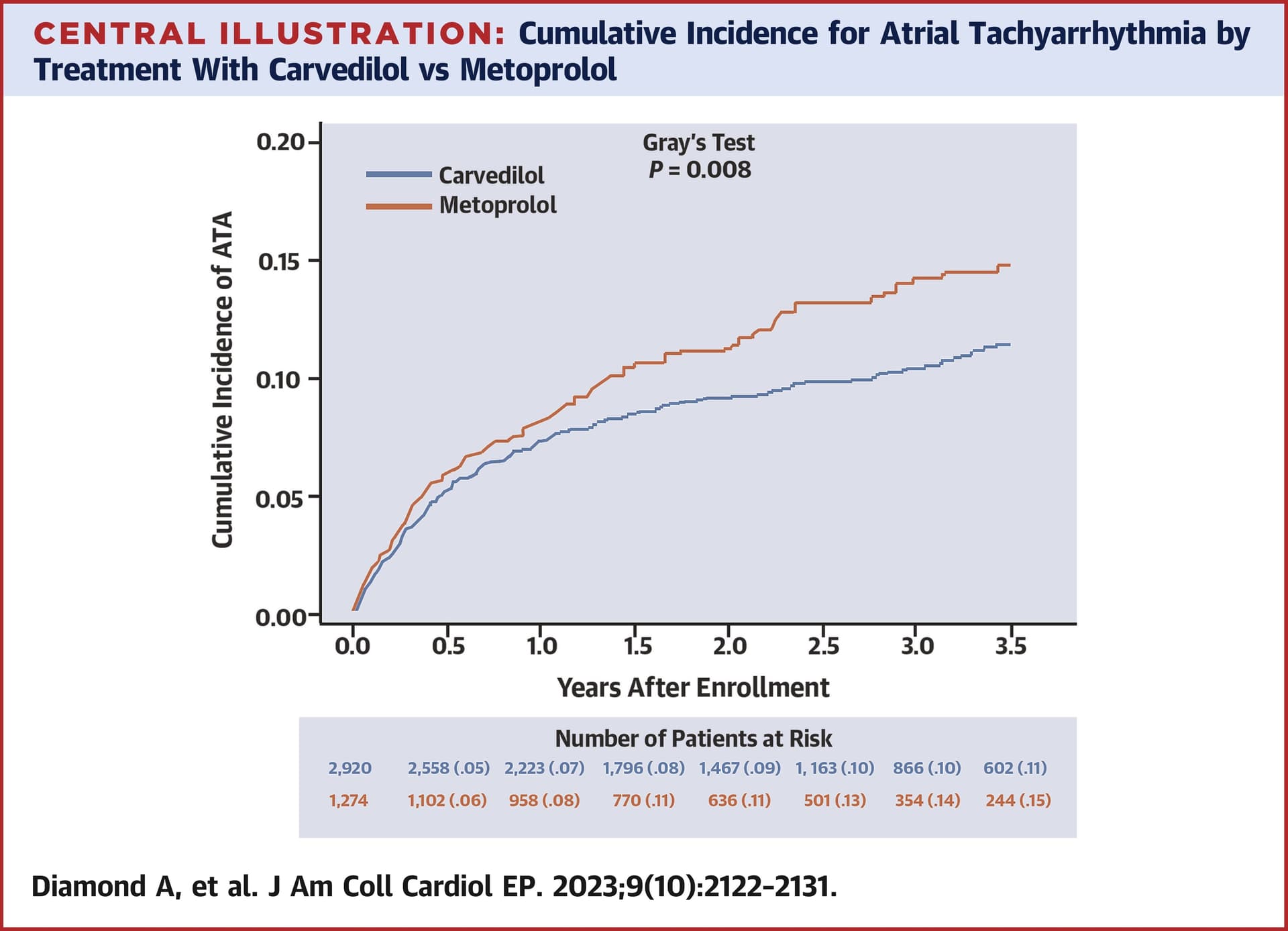
Carvedilol is better than metoprolol for arrhythmias: Effect of Carvedilol vs Metoprolol on Atrial and Ventricular Arrhythmias Among Implantable Cardioverter-Defibrillator Recipients 2023
It also works well in atrial fibrillation:
- Which should you choose for post operative atrial fibrillation, carvedilol or metoprolol? A systemic review and meta-analysis 2024
- Efficacy and safety of once-daily carvedilol in atrial fibrillation patients: a randomized, double-blind, placebo-controlled trial 2024
So, if someone needs a BB (for instance to lower HR), then carvedilol might be the best option. What do you think @Davin8r?
However here carvedilol, although the best performing beta-blocker, ranks below placebo (not sure it’s a great paper though): EFFECT OF ANTIHYPERTENSIVE AGENTS ON RISK OF NEW-ONSET AND RECURRENT ATRIAL FIBRILLATION: A NETWORK META-ANALYSIS OF RANDOMIZED CONTROLLED TRIALS 2024
Ramipril + HCTZ is the best combination to reduce the risk of new-onset and recurrent AF among patients with hypertension, diabetes, or AF. However, not all combinations were tested (due to a lack of trials). Telmisartan was the best-performing single agent. As ramipril + HCTZ = 0.95 vs 0.66 for ramipril and perindopril + indapamide = 0.51 vs 0.34 for perindopril, I can imagine that telmisartan + indapamide would be even better than ramipril + HCTZ.
Carvedilol and nebivolol have always been the two “different” beta-blockers that stood out from the others. I did lit reviews on them about 15 years ago to help determine which one I wanted to try, also tried both of them. Since nebivolol gave me zero side effects while carvedilol made me tired and gave me a headache, I went with nebivolol.
Based on your updated lit search, carvedilol certainly has potential, but yes those studies on afib do seem contradictory. The last one seems the most applicable for afib in particular to the general population of people with afib, since the previous studies you mentioned were under more specific conditions (post surgical patients, patients with implantable defibrillators). One of the studies you mentioned only showed that carvedilol effectively helped control heart rate during afib and didn’t address afib prevention.
So I’m still aiming to completely get off nebivolol and control BP with telmisartan alone (along with empagliflozin as my “diuretic”), which hopefully also will decrease future risk of afib.
Empagliflozin is NOT a diuretic, see the recent: SGLT2 Inhibition: Neither a Diuretic nor a Natriuretic commenting on Water Conservation Overrides Osmotic Diuresis During SGLT2 Inhibition in Patients With Heart Failure.
Many investigators attribute the beneficial effects of SGLT2i to the drugs’ natriuretic diuretic or osmotic diuretic effects. The underlying assumption is that, similar to diuretics, SGLT2i increase urine volume and thereby exert beneficial effects on cardiac workload and myocardial energy expenditure, especially in states of cardiac congestion. However, the effects of SGLT2i on solute-driven urine volume formation are different from those of traditional diuretics. The observed natriuretic, osmotic diuretic effects of SGLT2i are often short-lasting, modest, or absent. Retrospective analysis of the currently available data suggests that SGLT2 inhibition does not produce durable decongestion.
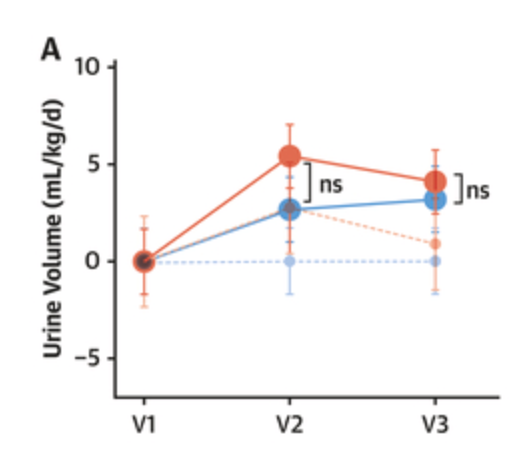
Despite the significant early and persistent increase in urine solutes (Figure 3A) and glucose (Figure 3D) excretion, treatment with dapagliflozin resulted in a statistically nonsignificant early increase in urine volume (mean difference 2.8 mL/kg/d; 95% CI: −1.97 to 7.48; P = 0.25), which diminished after 4 weeks (0.9 mL/kg/d; 95% CI: −3.83 to 5.62; P = 0.7)
These findings suggest that SGLT2i may not improve cardiac health status by osmotic diuretic decongestion. An alternative “nutrient deprivation signaling/autophagy hypothesis” assigns the beneficial effects of SGLT2i to reprogramming of mitochondrial function. Similar switches in mitochondrial fuel utilization occur in “aestivation,” an evolutionary conserved survival strategy in response to combined energy and water deficit.The observed ≈150 kcal/d loss of glucose fuel into the urine (Figure 3D) not only requires adaptive energy conservation to prevent an energy deficit but also triggers physiological water conservation to counteract the osmotic diuretic effect of glucosuria and prevent dehydration (Figure 4C). As an extension to the “nutrient deprivation signaling/autophagy hypothesis,” we suggest that SGLT2 inhibition may result in a biomimicry of aestivation metabolism and thereby improve health span.
Sorry, I meant to put “diuretic” in quotes. Empagliflozin as a replacement for a diuretic BP med to hopefully reduce risk of afib and help to keep BP under control.
Yes, but I don’t know if SGLT2i can replace a diuretic BP med: “In normotensive, nonobese individuals, SGLTi-induced changes in BP and weight are minimal to absent.” ( https://www.sciencedirect.com/science/article/pii/S0033062023001068?via%3Dihub#bb0050 )
Ok but I’m not normotensive without meds, which is why I’m taking BP meds ![]()
Ah OK. What’s your BP without meds?
No idea. I’ve been on one BP med or another for 20+ years.
What is it now then? What I understand is that if your SBP is below 140 mmHg (whether with or without meds), it won’t decrease more on SGLT2i. (Contrary to proper BP lowering diuretics.)
117/78 at the moment, but I’ve been on empa for months. Was 111/71 yesterday when I checked. Planning to stop the tiny dose of nebivolol 2.5 mg and go from 40mg to 80mg telmisartan by the end of the week. Not going to bother with adding a true diuretic unless BP doesn’t remain well controlled.
Another position paper against the return of beta-blockers in the recent European guidelines: For Debate: The 2023 European Society of Hypertension guidelines - cause for concern 2024
Originally, the beta-blockers were equally ranked alongside the other antihypertensive drug classes. Things changed when two major long-term randomized controlled trials, ASCOT-BPLA and LIFE showed that the patients receiving the beta-blockers based regimes suffered 25–30% more strokes than those receiving a calcium channel blocker based regime or an angiotensin receptor blocker based regime. The inferiority of the beta-blockers at stroke prevention was not due to differences in blood pressure control during the follow-up period in both trials. The 2023 European Society of Hypertension (ESH) guidelines still argue in favour of beta-blockers that their clinical inferiority was simply to lesser blood pressure reduction rather than class effect. The analysis argues that the return of beta-blockers as a first-line option for the management of uncomplicated hypertension by the ESH is a cause for concern and should be reconsidered.
Given this evidence, it seems strange that the new 2023 ESH guidelines reinstate the beta-blockers as suitable first-line therapy for hypertension, although with caveats in other paragraphs. Why is this volte-face based on no new evidence? The message is confusing, and we profoundly disagree. We are not alone in these views; Messerli, Bangalore and Mandrola share many of our sentiments in a similarly detailed review.
Sadly, all the guideline committees (from the USA, the UK and Europe) largely comprise hospital-based specialists. In contrast, most patients with hypertension are managed exclusively in primary care settings because they are less likely to have cardiovascular complications of hypertension. Hospital-based hypertension specialists are more likely to encounter more complex cases many of whom may have suffered the vascular complications of hypertension. The re-endorsement of the beta-blockers for uncomplicated hypertension might not have happened if more primary care physicians had had greater input into the preparation of the guidelines.
To some extent, arguments about first-line drugs are of only limited use as, in most patients, double or triple therapy is necessary to bring the BP under control. But even then, the beta-blockers are only required if there is concomitant heart disease. In primary healthcare, such patients are in the minority. Hence, the return of beta-blockers as a first-line option for the management of uncomplicated hypertension by the ESH is a cause for concern and should be reconsidered.
Beta blockers are good for aortic stenosis. I have moderate regurgitation/stenosis so I am going to remain on 5mg nebivolol I think, even though I don’t have high BP without it.
Arterial Hypertension in Aortic Valve Stenosis: A Critical Update - PMC (nih.gov)
" If blood pressure is not yet controlled by RAAS blocking, the addition of a beta-blocker (BB) should be considered; among these, metoprolol has the greatest literature evidence, showing not only an improvement in hemodynamic and metabolic performance but also a reduction in mortality in patients who already presented with coronary artery disease [77,78].
BB therapy was also linked to decreased rates of cardiac and all-cause mortality, along with sudden cardiac death, and was not linked to an increased incidence of heart failure prior to AVR [79]."
I have been taking metoprolol prescribed by a locally imminent cardiologist for more than a decade. I recently (several months ago) added telmisartan (self-prescribed).
People may wonder why I do these things. It is because I am on a quest to optimize what is presently known to be optimal in the form of things I can measure.
I like Peter Attia’s approach to optimization, but I could not possibly adhere to his diet.
So I am trying to optimize, as others in the forum are doing, by adjusting my supplements, medications, and a diet that I can adhere to optimize my blood markers and blood pressure. My advice to naysayers is; just don’t do it. I am not advising anything, just reporting what I do and my own observations.
Yes beta blockers might be good for some specific indications, for instance, arrhythmias. However, for essential hypertension and for neuroprotection, they’re not recommended as of today.
After being on Telmisartan 80 for another month and a half after writing this, I definitely feel a positive affect on my body composition. I do take other stuff like rapa/jardiance known to affect fat loss, so it could be synergy or other compounds. But since being on Telmisartan 80, I’ve noticed some fat loss.
I don’t know what else I will add to get my BP where I want it. I have light CKD so its IMO I’ve read its even better for me to lower my readings. The thing about this is, I take high dose melatonin which can have a blood pressure lowering affect, but my readings at night after the Telmisartan 80 are dead at the hypotension line (Is it 90/60?), with all I take. Its just that because I take caffeine and dont have the best diet and even smoke intermittently (hopefully that doesn’t take away my rapamycin.news pass) my day time blood pressure can get to the 120 systolic level which I want down.
adding 5/10 of amlodipine at night might push that hypotension over the edge