Peripheral artery disease (PAD) is the narrowing of the arteries that carry blood to the lower extremities. PAD has been traditionally associated with atherosclerosis. However, recent studies have found that thrombotic events triggered by medial arterial calcification (MAC) is the primary cause of chronic limb ischemia below the knee. MAC is localized around the elastic fibers surrounding smooth muscle cells (SMCs) in arteries. Matrix GLA protein (MGP) binds circulating calcium and prevents hydroxyapatite mineral deposition, while also modulating pro-osteogenic signaling by attenuating BMP-2-mediated activation of Runx2 gene expression. Mgp-/- mice develop severe MAC and die around 8 weeks after birth due to aortic rupture or heart failure. We previously discovered a rare genetic disease Arterial Calcification due to Deficiency of CD73 (ACDC), in which patients present with extensive MAC in their lower extremity arteries. Using a patient-specific induced pluripotent stem cell model, we found that rapamycin inhibited calcification. Here we investigated whether rapamycin could reduce MAC in vivo using the Mgp-/- murine model. Mgp+/+ and Mgp-/- mice received 5mg/kg rapamycin or vehicle. Calcification content was assessed via microCT, and vascular morphology and extracellular matrix content were assessed histologically. Immunostaining and western blot analysis were used to examine SMC phenotype and extracellular matrix content. Rapamycin prolonged Mgp-/- mice lifespan, decreased mineral density in the arteries, maintained SMC contractile phenotype, and improved vessel structure, however, calcification volume was unchanged. Mgp-/- mice with SMC-specific deletion of Raptor or Rictor did not recapitulate treatment with rapamycin. These findings suggest rapamycin promotes beneficial vascular remodeling in vessels with MAC.
Noticing the dose used, 5mg/kg, about 360mg for a human. Not sure how that would be employable. But, an interesting study.
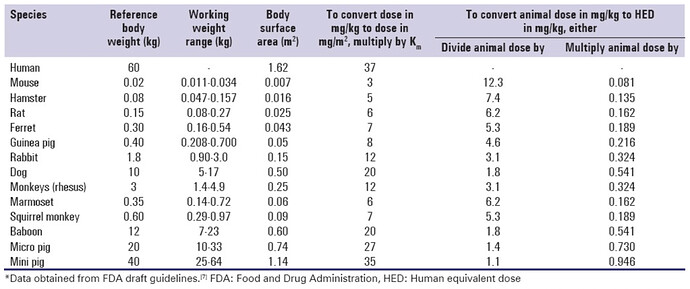
No, thats not how you calculate dose equivalence across species.
That 5mg/kg dose (assuming its based on weight of animal, and not as the ITP does which is per kg of feed), would translate to: (5 / 12.3) * 70kg (for human avg. weight) = 28mg.
Still high, but not as insanely high as 360mg.
Here are some details on how you do dose conversion and how it maps in terms of blood levels:
From this document:
I think daily dosing in mice is roughly equivalent to about once every 4 days or so in human terms given the speed that mice metabolize rapamycin is about 4 times faster.
| Sirolimus Dose |
Mouse mg/kg/day Dose |
Mouse: Blood/Sirolimus Level |
Human mg/kg/day Dose |
Dose for 60kg Human | Daily Dose adjusted for longer half-life (/4) |
|---|---|---|---|---|---|
| 4.7ppm | ∼2.24 | 3 to 4 ng/mL | 0.182 mg/kg | 10.92 mg | 2.73 mg |
| 14ppm | ~6.67 | 9-16 ng/mL | 0.542 mg/kg | 32.54 mg | 8.135 mg |
| 42ppm | ~20 | 23-80 ng/mL | 1.626 mg/kg | 97.56 mg | 24.39 mg |
| 126ppm | ~60 | 4.878 mg/kg | 292.68 mg | 73.17 mg | |
| 378ppm | ~180 | 45 to 1800 ng/mL | 14.634 mg/kg | 878.04 mg | 218 mg |
| Sirolimus Dose |
mg/kg/day Dose |
Blood/Sirolimus Level |
Male Median LS Increase | Female Median LS Increase | |
| 4.7ppm | ∼2.24 | 3 to 4 ng/mL | 3% | 16% | |
| 14ppm | ~6.67 | 9-16 ng/mL | 13% | 21% | |
| 42ppm | ~20 | 23-80 ng/mL | 23% | 26% |
Based on the FDA animal to human dosing conversion guide here.
Note: ½ life for sirolimus in mice is approx. 15 hours, vs. approx. 62 hours in humans. So, mice metabolize sirolimus approximately 4 times faster than humans.
Totally forgot that! Thanks for posting the specifics.
Related reading (for this thread):
Using factors like blood flow and stiffness components to quantify brain health could help lead to earlier detection of neurological diseases like Alzheimer’s, which currently has no cure. Some studies reveal that people with Alzheimer’s show softening in the hippocampus. One hypothesis is that in early Alzheimer’s, a reduction in blood flow could lead to this change in the brain, Neher says.
“The study focused on the basic science question of how blood flow and brain stiffness are related,” she says. “It would be interesting to eventually apply this to a patient population by collaborating with UW Medicine. We want to better understand this link between brain stiffness and blood flow, and come up with diagnostic criteria.”