TORC1 and TORC2 are brothers. TORC2 is always trying to get TORC1 worked up, usually by convincing their cousin Akt to switch out TORC1’s decaf for a regular cup of coffee. TORC1 isn’t stupid, though. Usually, he keeps this in check by calming his brother down and getting their dog, S6K, to play with him. TORC1 also knows that their cousin, Akt, is only a trouble maker after she eats a lot of candy. So, he makes sure there isn’t any around. He also enlists their health-conscious grandmother to check for candy, too. But, if TORC1 wakes up tired, he doesn’t have the energy to calm his brother down, forgets to have their dog, S6K, go play with him, forgets to check the house for candy, and doesn’t remind his grandmother to check either. So, TORC2 gets excited, convinces sugar-rush Akt to switch out TORC1’s decaf and now as his morning grogginess wears off, TORC1 suddenly surges back to life.
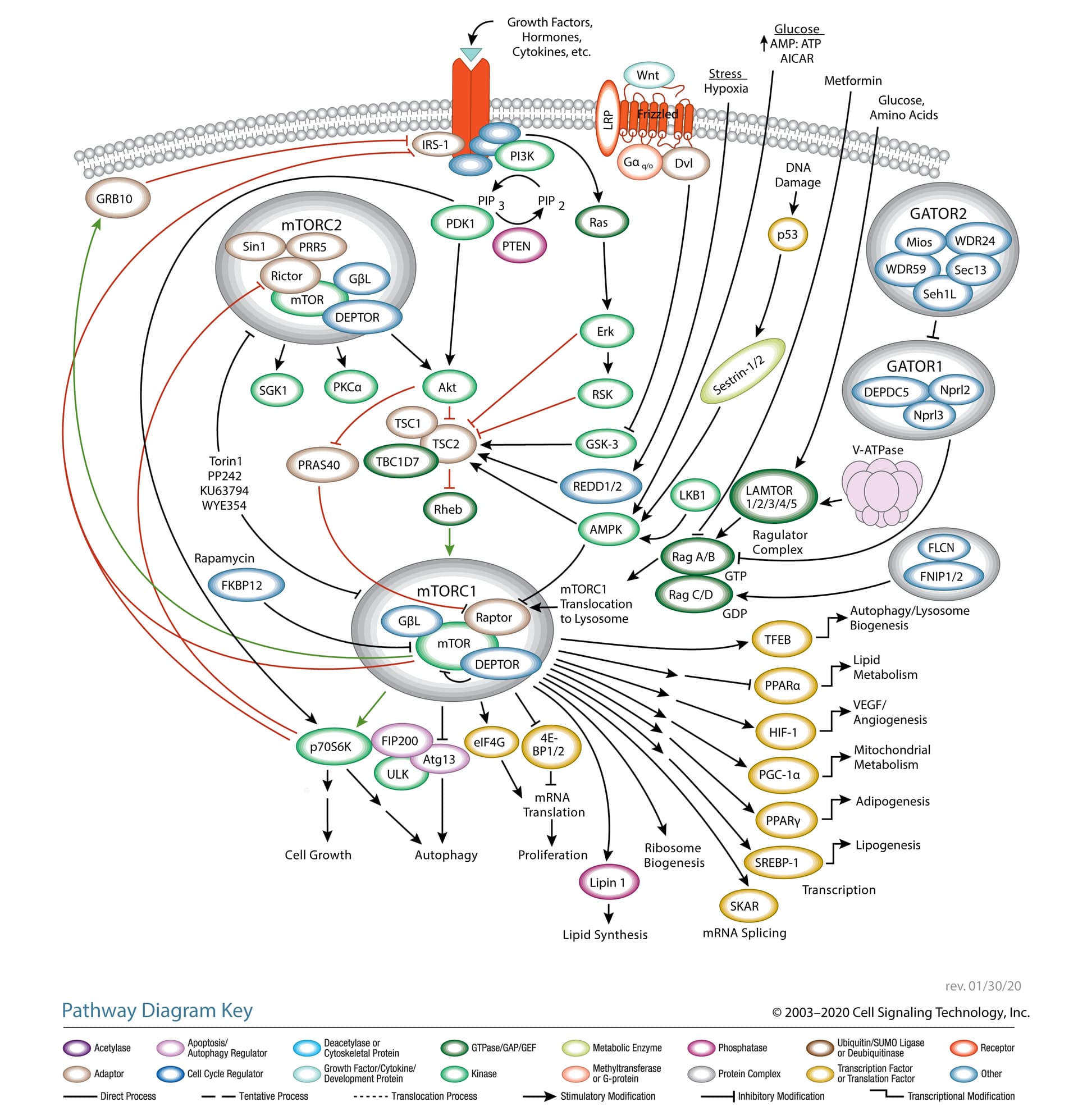
To provide some biological context to that little story, I’ve slightly modified a diagram of the mTOR pathway from Cell Signaling Technologies. On first glance, it’s a lot. But follow the colors: green means activation and red means inhibition. I haven’t modified the right side of the diagram, so we’ll focus on the left.
Start with mTORC1 in the lower left. It activates p70S6K. Trace the red (inhibition) lines upward and you’ll see that p70S6K inhibits Rictor (a central component of mTORC2) and IRS-1 (insulin receptor substrate 1).
Now go back to mTORC1. You’ll see that mTORC1 also inhibits IRS-1. You’ll also see a green line extending to GRB10, which then goes on to inhibit IRS-1.
Now, here’s the same story with some added biological detail:
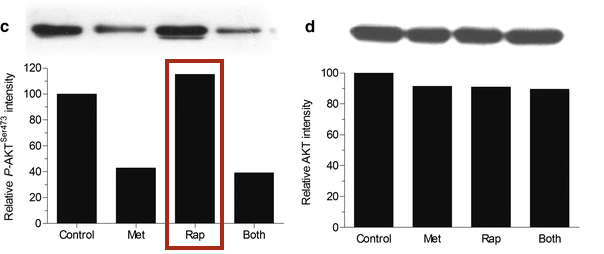
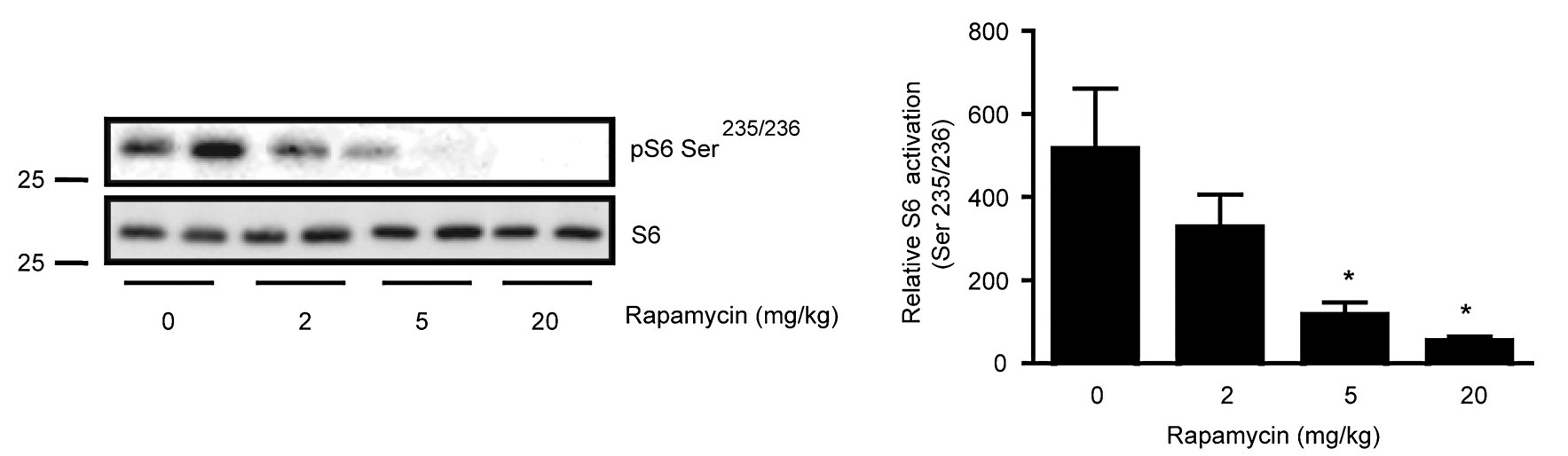
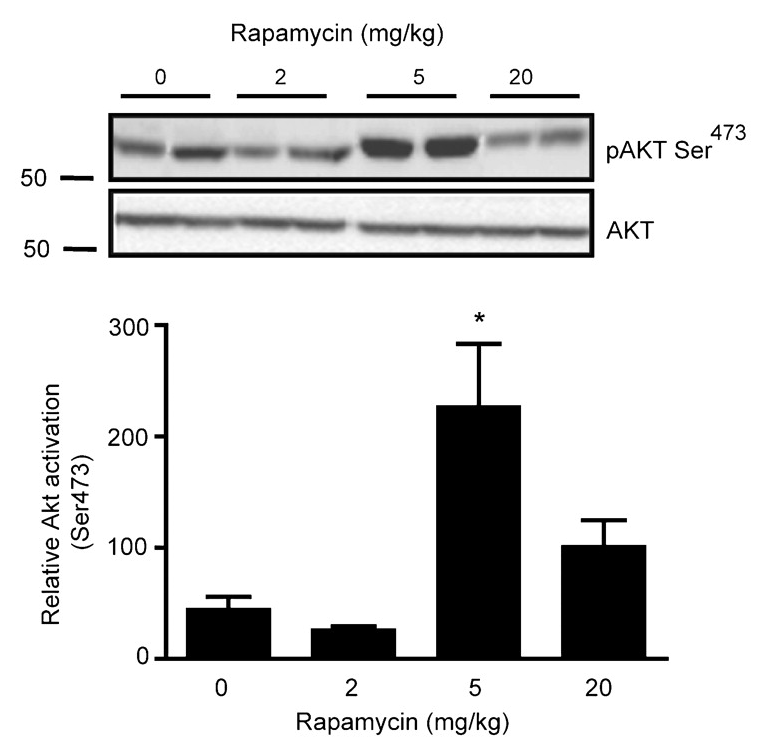
TORC1 and TORC2 are brothers. TORC2 is always trying to get TORC1 worked up, usually by convincing their cousin Akt to switch out TORC1’s decaf [↓ PRAS40 ↓ TSC2] for a regular cup of coffee [↑ Rheb]. TORC1 isn’t stupid, though. Usually, he keeps this in check by calming his brother down [↓ Rictor ↓ mTORC2] and getting their dog, S6K, to play with him [↓ Rictor ↓ mTORC2]. TORC1 also knows that their cousin, Akt, is only a trouble maker after she eats a lot of candy. So, he makes sure there isn’t any around [↓ IRS-1]. He also enlists their health-conscious grandmother [↑ GRB10] to check for candy, too [↓ IRS-10]. But, if TORC1 wakes up tired [Rapamycin —| mTORC1], he doesn’t have the energy to calm his brother down [↑ Rictor ↑ mTORC2], he forgets to have their dog, S6K, go play with him [↑ Rictor ↑ mTORC2], forgets to check the house for candy [↑ IRS-1], and doesn’t remind his grandmother [↓ GRB10] to check either [↓ IRS-1]. So, TORC2 gets excited, convinces sugar-rush Akt to switch out TORC1’s decaf and now as his morning grogginess wears off [Rapamycin ↓ mTORC1 ↑], TORC1 suddenly surges back to life.
It’s important to keep in mind that mTORC1 and mTORC2 participate in a regulatory loop. Most cell signaling works like that, through complicated loops. So, as mTORC1 activity surges back it will begin inhibiting mTORC2 again, which will then decrease the Akt-mediated increase in mTORC1, allowing mTORC1 to decrease, which will then release some of its inhibition of mTORC2, and so on… until the system reaches an equilibrium.