Kind of a nasty, defensive response from a scientific researcher to be honest with you.
Personally, I’m persuaded that in the context especially of AD, rapamycin is probably preventative but not curative. I’d be cautious using it in a patient diagnosed with AD until we know more.
The Takeaways from the Lancet journal article recommended by Tim Sergeant above:
Dementia prevention, intervention, and care: 2020 report of the Lancet Commission
https://www.thelancet.com/article/S0140-6736(20)30367-6/fulltext
Key messages
-
Three new modifiable risk factors for dementia
-
New evidence supports adding three modifiable risk factors—excessive alcohol consumption, head injury, and air pollution—to our 2017 Lancet Commission on dementia prevention, intervention, and care life-course model of nine factors (less education, hypertension, hearing impairment, smoking, obesity, depression, physical inactivity, diabetes, and infrequent social contact).
-
Modifying 12 risk factors might prevent or delay up to 40% of dementias.
-
Be ambitious about prevention
-
Prevention is about policy and individuals. Contributions to the risk and mitigation of dementia begin early and continue throughout life, so it is never too early or too late. These actions require both public health programmes and individually tailored interventions. In addition to population strategies, policy should address high-risk groups to increase social, cognitive, and physical activity; and vascular health.
Specific actions for risk factors across the life course:
- Aim to maintain systolic BP of 130 mm Hg or less in midlife from around age 40 years (antihypertensive treatment for hypertension is the only known effective preventive medication for dementia).
- Encourage use of hearing aids for hearing loss and reduce hearing loss by protection of ears from excessive noise exposure.
- Reduce exposure to air pollution and second-hand tobacco smoke.
- Prevent head injury.
- Limit alcohol use, as alcohol misuse and drinking more than 21 units weekly increase the risk of dementia.
- Avoid smoking uptake and support smoking cessation to stop smoking, as this reduces the risk of dementia even in later life.
- Provide all children with primary and secondary education.
- Reduce obesity and the linked condition of diabetes. Sustain midlife, and possibly later life physical activity.
- Addressing other putative risk factors for dementia, like sleep, through lifestyle interventions, will improve general health.
- Tackle inequality and protect people with dementia
- Many risk factors cluster around inequalities, which occur particularly in Black, Asian, and minority ethnic groups and in vulnerable populations. Tackling these factors will involve not only health promotion but also societal action to improve the circumstances in which people live their lives. Examples include creating environments that have physical activity as a norm, reducing the population profile of blood pressure rising with age through better patterns of nutrition, and reducing potential excessive noise exposure.
- Dementia is rising more in low-income and middle-income countries (LMIC) than in high-income countries, because of population ageing and higher frequency of potentially modifiable risk factors. Preventative interventions might yield the largest dementia reductions in LMIC.
And don’t forget about this
We see vitamin d deficiency Frequently in the obese.
For those wanting to take a deeper dive, Dale Bredesen, both an AD researcher and clinician, has a couple of prevention books out.
Still troubling now that we ApoE4’s have the ability to check if we have amyloid plaque in our blood (I do). If there are no signs of cognitive decline yet and all lifestyle recommendations are being followed then am I still in preventative stage or does amyloid plaque in my blood mean Rapamycin treatment is contraindicated?
Besides the many unanswered translation questions, WHEN to start prevention? When symptomatic, it’s too late, and classic amyloid dementia shows signs of amyloid build decades before symptoms. Using an average symptomatic age of 65, then would need to start 40 yrs old? YOUNGER?
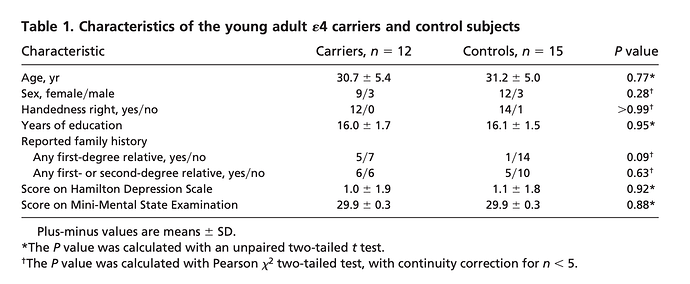
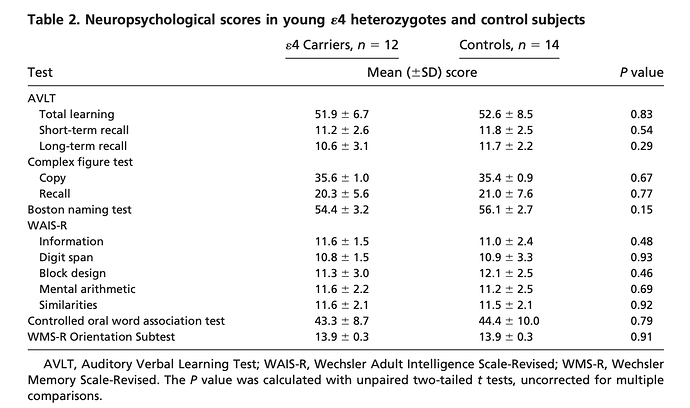
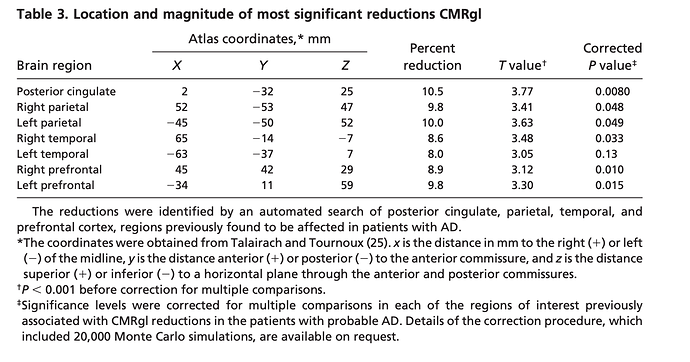
Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia
“Fluorodeoxyglucose positron emission tomography (PET) studies have found that patients with Alzheimer’s dementia (AD) have abnormally low rates of cerebral glucose metabolism in posterior
cingulate, parietal, temporal, and prefrontal cortex. Apolipoprotein E genotypes were established in normal volunteers 20–39 years of age. Like previously studied patients with probable AD and late-middle aged 4 carriers, the young 4 carriers had abnormally low rates of glucose metabolism bilaterally in the posterior cingulate, parietal, temporal, and prefrontal cortex. Carriers of a common Alzheimer’s susceptibility gene have functional brain abnormalities in young adulthood, several decades before the possible onset of dementia”
There is a spectrum of associated amyloid across age as well.
Besides genetic and epigenetic risks, there is evidence one can have congenital risk (smaller hippocampal sub regions) right out of the gate, at birth.
Clearly, prevention by ALL interventions starts as early as possible!!
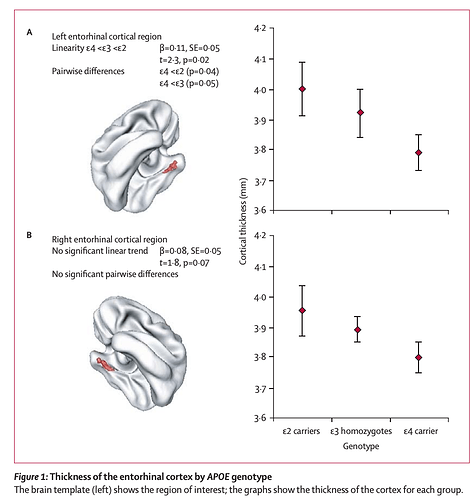
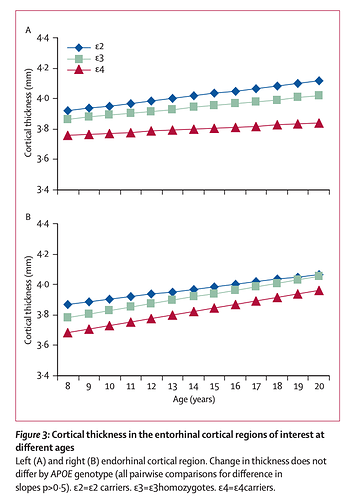
Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study
https://sci-hub.se/https://doi.org/10.1016/S1474-4422(07)70106-0
Methods: 239 healthy children and adolescents were genotyped and had repeated neuroanatomic MRI (total 530 scans). Mixed model regression was used to determine whether the developmental trajectory of the cortex differed by genotype.
Findings: Cortical thickness of the left entorhinal region was signifi cantly thinner in ε4 carriers than it was in non-ε4 carriers. There was a significant stepwise increase in cortical thickness in the left entorhinal regions, with ε4 carriers having the thinnest cortex and ε2 carriers the thickest, with ε3 homozygotes occupying an intermediate position. Neuroanatomic effects seemed fixed and non-progressive, with no evidence of accelerated cortical loss in young healthy ε4 carriers.
Interpretation: Alleles of the apolipoprotein E gene have distinct neuroanatomic signatures, identifiable in childhood. The thinner entorhinal cortex in individuals with the ε4 allele might contribute to risk of Alzheimer’s disease.
Wow, amazing (and tough) question.
Are you part of a trial re getting amyloid blood levels tested? Do you have one time stamp result, or trending?
Has anyone told you your amyloid blood level and risk level?
Have you had any detailed brain imaging, like FDG-PET for cerebral glucose metabolism and/or amyloid, or volumetric MRI looking at your sub brain regions for volume changes?
Can I ask how old you are?
Family history?
3/4 or 4/4?
Inducing Autophagy by Rapamycin Before, but Not After, the Formation of Plaques and Tangles Ameliorates Cognitive Deficits
Hi, thanks so much for your thoughtful response. I’m 62 years old, ApoE 3/4, and my father was diagnosed with Alzheimer’s at 70 and passed at 81. He was also ApoE 3/4 but he was a smoker, did not exercise much and ate a lot of Oreos, lol, but was a brilliant man.
Yes, I’m in a study, the AHEAD study, but just have lab work done so far. No level or trend on plaque in blood just a yes/no result. I’m due for cognitive testing week after next, and two Pet scans and an MRI if I pass cognitive tests. I have a normal brain MRI from a study I did 10 years ago.
I’m a patient of Dr. Green and will reach out to him but I must say I found this entire thread about possibility of Trem2 down regulation from Rapamycin unsettling.
It’s an interesting but almost impossible question to answer. Consider that even now we’re not sure of the etiology of AD. Is it amyloid,tau,inflammation, or some combination? Is it a vascular issue or even herpes related? All of these theories have support and difficulties.
I’d rely on the best neurologist I could find specializing in AD.
I agree, any recommendations for top neurologists specializing in AD, and open to new thinking, who are currently taking new patients?
Re @rapadmin posted Inducing Autophagy by Rapamycin Before, but Not After, the Formation of Plaques and Tangles Ameliorates Cognitive Deficits (2011)
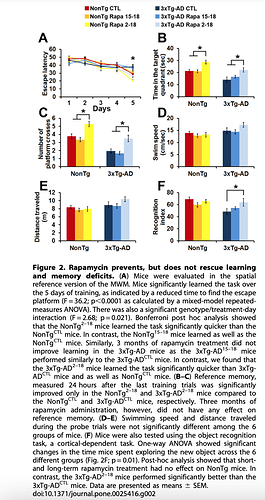
Good fundamental paper looking at a mouse model of amyloid and Rapamycin intervention, including control mice. Yes, it’s one of these transgenic mutant mice models, since mice don’t get amyloid like humans. Rapamycin administration, earlier in life ameliorates cognitive dysfunction of aging, in both wild control and transgenic models. Rapamycin DOES cross the BBB, impacts mTOR, and reduces cognitive decline. "Rapamycin prevents, but does not rescue learning and memory deficits"
This study did NOT measure levels of Rapamycin in the brain. But they did measure pmTOR, showing significant decline in a direct measurement of mTOR inhibition.
Is this fundamentally therapeutic translatable, and how to even measure in humans?! Looks like 30-50% mTOR inhibition in the brain is a necessary signal. We talk about “turning off mTOR”, yet we might need “some constant level” mTOR reduction in the brain for therapeutic efficacy?? I think this “off logic” is misguided rationale.
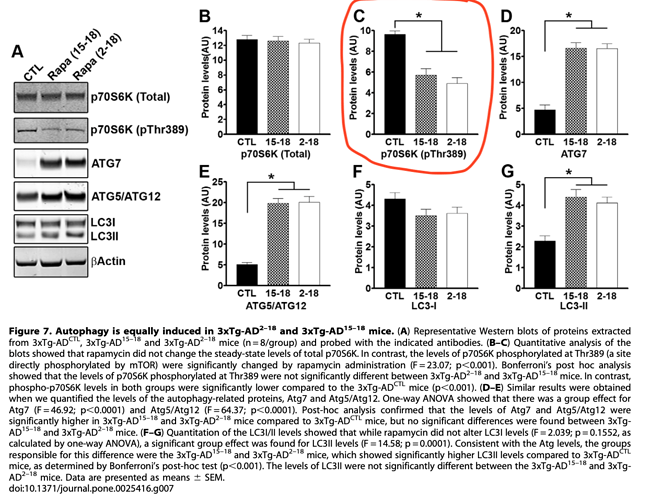
“Overall, our data show that under the conditions used here, facilitating autophagy induction prophylactically has beneficial effects on AD-like pathology; however, once plaques and tangles are well established, increasing autophagy induction is not sufficient to rescue AD-like pathology and the associated cognitive deficits”
“The role of autophagy in AD is controversial. For example, Nixon and colleagues have shown that in AD brains there is accumulation of autophagosomes. Additionally, they showed that autophagosomes may be another source of Ab generation, suggesting that interventions aimed at further increasing autophagy induction in AD may actually exacerbate the Ab pathology. In contrast, Wyss-Coray and colleagues have shown that increasing autophagy induction decreases Ab pathology in an animal model of AD. Data in apparent contradiction to each other have been also reported by others. Our data are compatible with both views as we show that increasing autophagy induction prior to the development of AD like pathology in the 3xTg-AD mice reduces the levels of soluble Ab and tau and the formation of thioflavin-positive plaques. In contrast, we show that if autophagy is induced after mature plaques and tangles are formed, no changes in Ab, tau or cognitive deficits are detected. We suggest that increasing autophagy induction may be a valid therapeutic strategy for AD if the intervention occurs early in the development of the disease. In contrast, once the neuropathology is well-established, increasing autophagy induction alone may not be sufficient to ameliorate the neuropathological phenotype and different approaches should be considered. Toward this end, recently it has been shown that reversing autophagy dysfunction by increasing lysosomal function, is a good strategy in AD”
We still do NOT understand the true upstream etiology of AD, but clearly once aggregate plaques and tangles are formed (in humans, extending translation of this mouse model), these don’t appear reversible and disrupt cognitive signalling.
Regarding timing and efficacy of Rapamycin administration to slow cognitive decline, that’s a very difficult question to answer.
It would depend on amyloid and plaque load, and some “arbitrary” cutoff definition re incipient acceleration from normal cognitive to MCI. This would require full brain scans for glucose hypometabolism, amyloid and tau. This is NOT standard imaging (very $$), typically only done in a trial setting, high risk patients. These data “might” assist with snapshot and risk trajectory assessment and whether Rapamycin is a proper risk/reward. This would need experienced AD focused neurologist up on the very latest treatment options. And even so, still a guess.
From:
Time to Amyloid Positivity and Preclinical Changes in Brain Metabolism, Atrophy, and Cognition: Evidence for Emerging Amyloid Pathology in Alzheimer’s Disease
Cerebrospinal fluid (CSF) biomarkers and positron emission tomography (PET) with tracers sensitive to Aβ, have made it possible to study the development of AD-related brain changes in the brain of living humans. However, it is not clear at what degree of Aβ pathology downstream AD-related changes start to occur. For example, is it necessary to have fully established Aβ pathology before any changes in metabolism can be detected, or can such changes occur much earlier, before conventional thresholds for Aβ+ are reached? Taken together, the studies discussed above suggest that there may be an early stage of abnormal Aβ-metabolism, when biomarker signs of Aβ-pathology start to appear and are related to other aspects of AD, even before they become Aβ+, i.e., prior to meeting the conventional criteria for preclinical AD. We call this stage of disease “emerging Aβ-pathology.”
Decline in delayed memory recall was estimated to begin accelerating 10 years before Aβ+, with global measures of cognition following 5–8 years later
From:
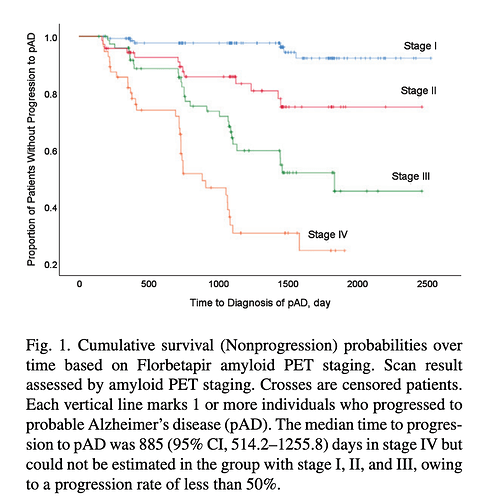
Quantitative Brain Amyloid Measures Predict Time-to-Progression from Amnestic Mild Cognitive Impairment to Alzheimer’s Disease
“To assess the prognostic impact of amyloid burden, a staging system based on the global SUVr of the PET scan was applied. We defined the stages as: stage I, negative amyloid scan; stage II, positive amyloid in 1st tertile; stage III, positive amyloid in 2nd tertile; and stage IV, positive amyloid in 3rd tertile.”
Conclusions: Large amyloid burden measured from amyloid PET scan could be a predictor of faster cognitive decline in aMCI patients.
1000%. Someone who can look at your complete profile, and you’re likely going to need the brain imaging to really assess your risk striation. The one CSF amyloid test is just a single data point, you will need more.
Sorry to hear about your dad. Is mom still alive, her status, genotype?
My mom has AD, still with us at 82, but went symptomatic around 72. Do not know her genotype. Good diet, no exercise, non smoker, overweight. Maternal AD confers higher risk. Mitochondrial DNA passes only from the mother.
The “normal brain MRI” dosen’t show much (unless some major volumetric loss), and you were a lot younger at 52.
And background on the drug/target:
https://www.alzforum.org/therapeutics/lecanemab
“BAN2401 is the humanized IgG1 version of the mouse monoclonal antibody mAb158, which selectively binds to large, soluble Aβ protofibrils”
Wishing you all the best in your journey.
Another challenge you “might have”, is neurologists don’t normally take you in unless you’re “symptomatic”. So you’ll have to find someone who sees the risk profile and wants to help you delay future decline.
This is complex… ran across this about how TREM2 inhibition may be a cancer therapy… so I don’t know what the trade-offs here are:
That’s interesting. So the same mechanism that could potentially exacerbate AD may inhibit, or even reverse, cancer growth.
This is interesting and fairly easy to do. Might want to consider it.
Thanks so much! I really appreciate you sending me that study. I will definitely look into it and step up my use of the 40hz light wave I use occasionally. I assume you saw the reference in the article you sent to Rapamycin use getting in the way of the FIR light working to help get rid of the amyloid plaque? Such a bummer, I really wanted Rapamycin to be my magic bullet!
It was very interesting that sun exposure ,using non melanoma skin cancers as a biomarker , has shown protection against AD.
I did see that rapamycin via mTOR inhibition inhibits the benefits of infrared. It’s a complicated mess.
Don’t forget though that you could use the infrared on those days when mTOR is least, or not at all , inhibited by the rapa.
It’s nice to know that all of my actinic keratoses are good for something.