Yet another model organism successfully treated with rapamycin with positive longevity results (in addition to nematodes, flies, mice, rats and marmoset monkeys; which typically see lifespan improvement of 15% to 30%). No other drug or compound has ever consistently increased healthy lifespan in such a broad range of species, repeatedly, by many different labs.
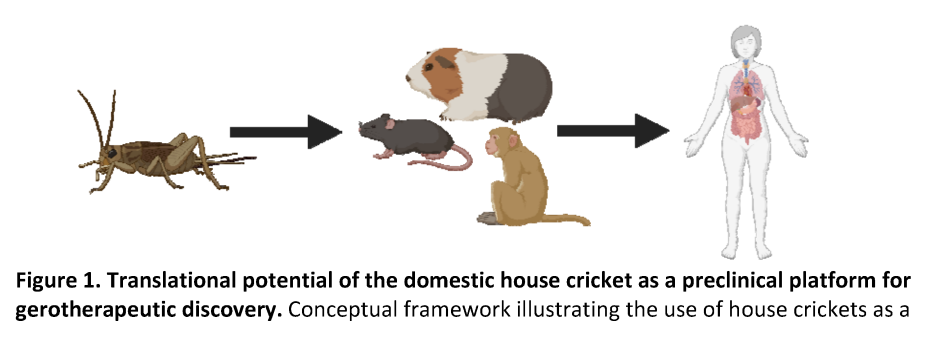
The house cricket (Acheta domesticus) is a promising preclinical geroscience model due to its short lifespan, low maintenance, age-associated functional decline, and responsiveness to geroprotective drugs. Continuous dosing with rapamycin, acarbose, and phenylbutyrate extends lifespan; whether intermittent dosing offers similar benefits remains unknown. We tested 274 sex-matched crickets given 2-week intermittent dosing of each drug starting at mid-age (8-weeks), followed by behavioral testing at 10-weeks (geriatric stage). Assays included Y-maze olfactory discrimination, open-field exploration, and treadmill performance. Locomotor gaits were identified by velocity-based K-means clustering (silhouette > 0.5). A subset was monitored for post-treatment survival using Kaplan-Meier analysis. Olfactory preference was preserved by all drugs (d = -1.82 to -1.28, P < 0.01), with strongest effects in rapamycin-treated individuals. Rapamycin-treated males matched or exceeded juvenile locomotor activity; phenylbutyrate reduced male activity (d = 1.49, P < 0.05) and acarbose increased walking-to-running ratios (d = -0.75, P < 0.05). Rapamycin increased central exploration and freezing (d = -1.55, P < 0.0001), while acarbose and phenylbutyrate increased peripheral freezing (d = -0.76, P < 0.05). Rapamycin and phenylbutyrate extended maximum running time (d = -2.30 to -1.32, P < 0.0001), with sex-specific jumping gains in rapamycin-treated females and acarbose-treated males. Post-treatment lifespan was prolonged by rapamycin (HR = 0.42, P < 0.001) and reduced by acarbose in females (HR = 2.92 to 3.03, P < 0.05). Intermittent rapamycin preserved survival, cognition, and locomotion, while acarbose and phenylbutyrate produced selective benefits, supporting A. domesticus as a scalable model for geroprotective drug discovery.
Full paper: https://www.biorxiv.org/content/10.1101/2025.08.25.671822v1?ct=
AI Summary:
Here’s a detailed summary of the paper:
The gerotherapeutic drugs rapamycin, acarbose, and phenylbutyrate extend lifespan and enhance healthy aging in house crickets.
Authors
Gerald Yu Liao, Jenna Klug, Sherwin Dai, Swastik Singh, Elizabeth Bae, Warren Ladiges (Univ. of Washington School of Medicine).
Background
-
Geroscience hypothesis: interventions targeting fundamental mechanisms of aging can delay multiple age-related diseases simultaneously.
-
Known drugs: Rapamycin (mTORC1 inhibitor), Acarbose (glucose metabolism modulator), and Phenylbutyrate (HDAC inhibitor/proteostasis enhancer) extend lifespan in mammals.
-
Problem: mammalian studies are costly and slow.
-
Solution: House crickets (Acheta domesticus) are proposed as a scalable invertebrate model because they have:
- Short lifespans, measurable aging phenotypes (cognition, locomotion).
- Conservation of aging mechanisms across species.
- Suitability for behavioral and histological assays.
Methods
-
Subjects: 274 sex-matched crickets.
-
Design: Intermittent 2-week mid-life (8 weeks) treatment with rapamycin (14 ppm), acarbose (1000 ppm), or phenylbutyrate (1000 ppm).
-
Testing age: 10 weeks (geriatric stage).
-
Assays:
- Y-maze (olfactory discrimination).
- Open field (exploration, freezing, locomotor behavior).
- Treadmill + jump test (running endurance, jump distance).
-
Data: Machine learning (k-means clustering of gait speeds), survival curves (Kaplan-Meier), and sex-stratified analyses.
Key Results
1. Cognition (Olfactory discrimination)
- All three drugs preserved odor preference compared to untreated aged crickets.
- Rapamycin showed the strongest effect, especially in males.
2. Locomotion (Open-field behavior)
- Rapamycin males maintained youthful levels of locomotion (speed and activity).
- Phenylbutyrate reduced activity in males.
- Acarbose increased walking-to-running ratios (less vigorous locomotion).
- Rapamycin-treated crickets froze more in the central arena—interpreted as exploratory pauses rather than frailty.
3. Induced locomotion (Treadmill & Jumping)
- Rapamycin and phenylbutyrate extended maximum running duration.
- Sex-specific benefits: rapamycin improved female jumping, acarbose improved male jumping.
- Jumping power overall was less responsive to intervention.
4. Body weight
- Acarbose-treated females gained significantly more weight than controls.
- Across all treatments, females gained more weight than males.
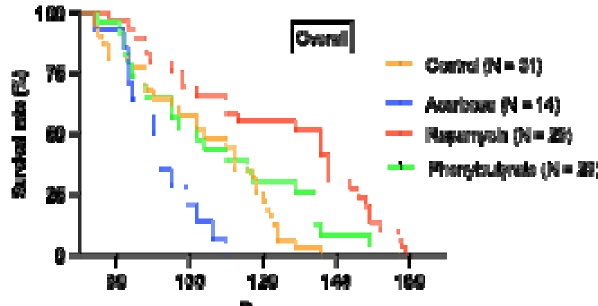
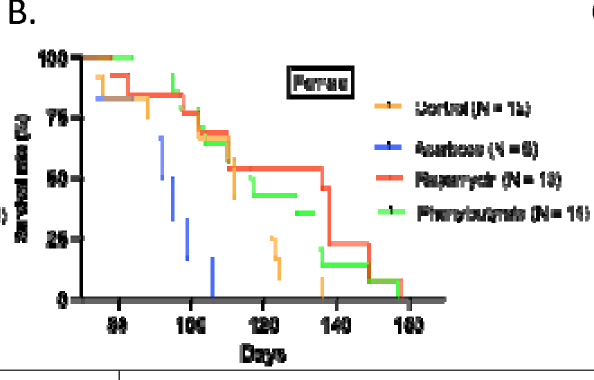
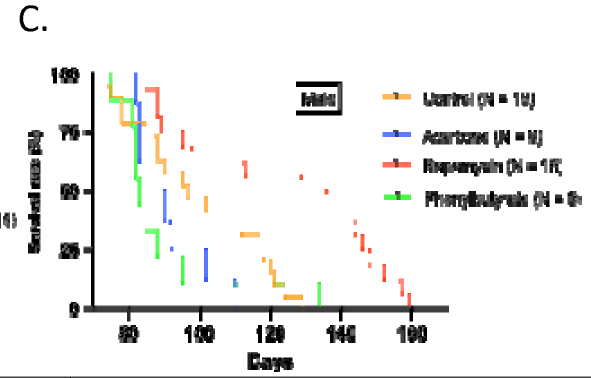
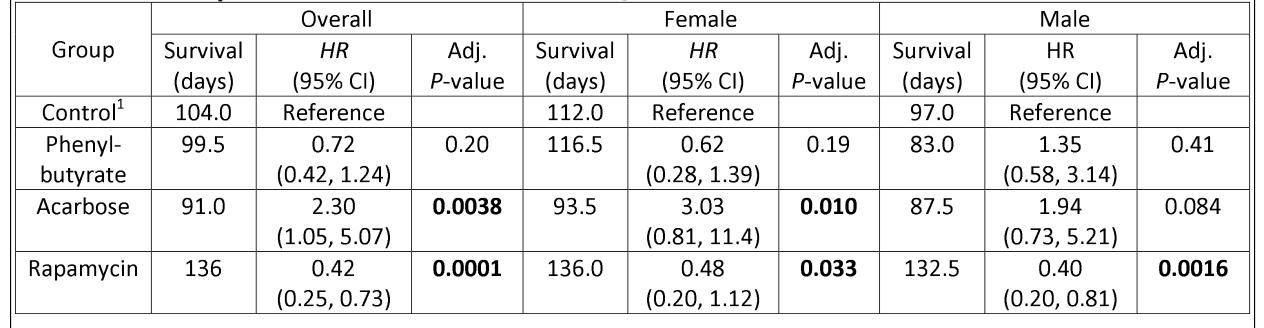
5. Lifespan
- Rapamycin extended lifespan post-treatment (HR 0.42, P < 0.001), especially in males.
- Acarbose reduced female lifespan (HR ~3.0, P < 0.01).
- Phenylbutyrate showed no significant survival benefit, though females lived longer than males.
Discussion
- Rapamycin: Most consistent benefit, preserving cognition, locomotion, and survival, even after short-term treatment.
- Acarbose: Mixed effects—modest cognitive benefit, but reduced female lifespan.
- Phenylbutyrate: Improved endurance and some cognition, but survival benefit was not robust.
- Sex-specificity: Drug effects differed by sex, echoing patterns seen in mice (e.g., rapamycin better in males, acarbose in males, phenylbutyrate in females).
- Implications: Intermittent drug dosing can remodel long-term aging outcomes, particularly with rapamycin.
Limitations & Future Directions
- Small behavioral sample sizes (need power simulations).
- Lack of fine-grained gait/kinematic analyses.
- No histological validation of neural/muscle tissue effects.
- Future work: combinatorial treatments (rapamycin + acarbose + phenylbutyrate), early-life dosing, and other geroprotectors (metformin, NAD⁺ precursors, SGLT2 inhibitors).
Conclusion
- House crickets are validated as a new preclinical model for geroscience.
- Rapamycin emerges as the strongest candidate, showing conserved benefits across species.
- Acarbose and phenylbutyrate show domain-specific or sex-specific benefits.
- The cricket model provides a rapid, low-cost, scalable system to screen geroprotective drugs before mammalian studies.