I kept searching mycotherapy §(medicinal mushrooms) for cancer prevention. The recurring combo for cancer prevention (as sell as cure adjuvant) seems to be:
- Turkey tail (Trametes or Coriolus versicolor)
- Reishi (Ganoderma lucidum).
My concern is whether it is acceptable in prevention to take them intermittently, for example, one or two months on and two off. For various reasons, including cost: high-quality powders and extracts are not inexpensive.
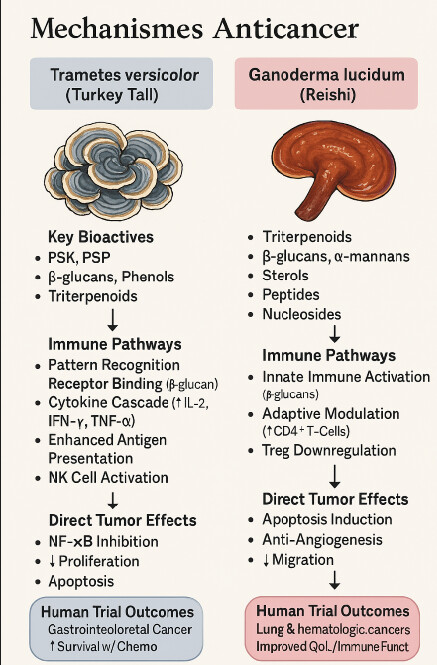
Here’s a concise, prevention‑focused snapshot of what the medical and pre‑clinical literature says about Coriolus versicolor (Trametes versicolor) and Ganoderma lucidum (Reishi) when the goal is reducing cancer risk rather than treating established disease.
 Biological rationale for prevention
Biological rationale for prevention
- Immune surveillance support:
- Anti‑inflammatory effects:
- Antioxidant activity:
- Direct anti‑proliferative actions:
 Selected preventive‑relevant findings
Selected preventive‑relevant findings
- Colorectal adenoma suppression: A water‑soluble extract from G. lucidum mycelia inhibited the development of colorectal adenomas in a small human study, suggesting possible chemopreventive activity in the colon.
- Immune modulation in healthy or at‑risk adults: Pilot trials and observational studies report increased NK‑cell activity and T‑cell subsets after supplementation, which could translate into improved cancer immunosurveillance.
- Synergistic potential: Reviews note that combining G. lucidum and C. versicolor may broaden the spectrum of bioactive compounds, potentially enhancing preventive effects through complementary mechanisms.
In summary: Laboratory and early human data suggest that Coriolus versicolor and Ganoderma lucidum may help reduce cancer risk by modulating immunity, lowering inflammation, and protecting against oxidative DNA damage. While promising, these findings need confirmation in robust, long‑term prevention trials before firm recommendations can be made.