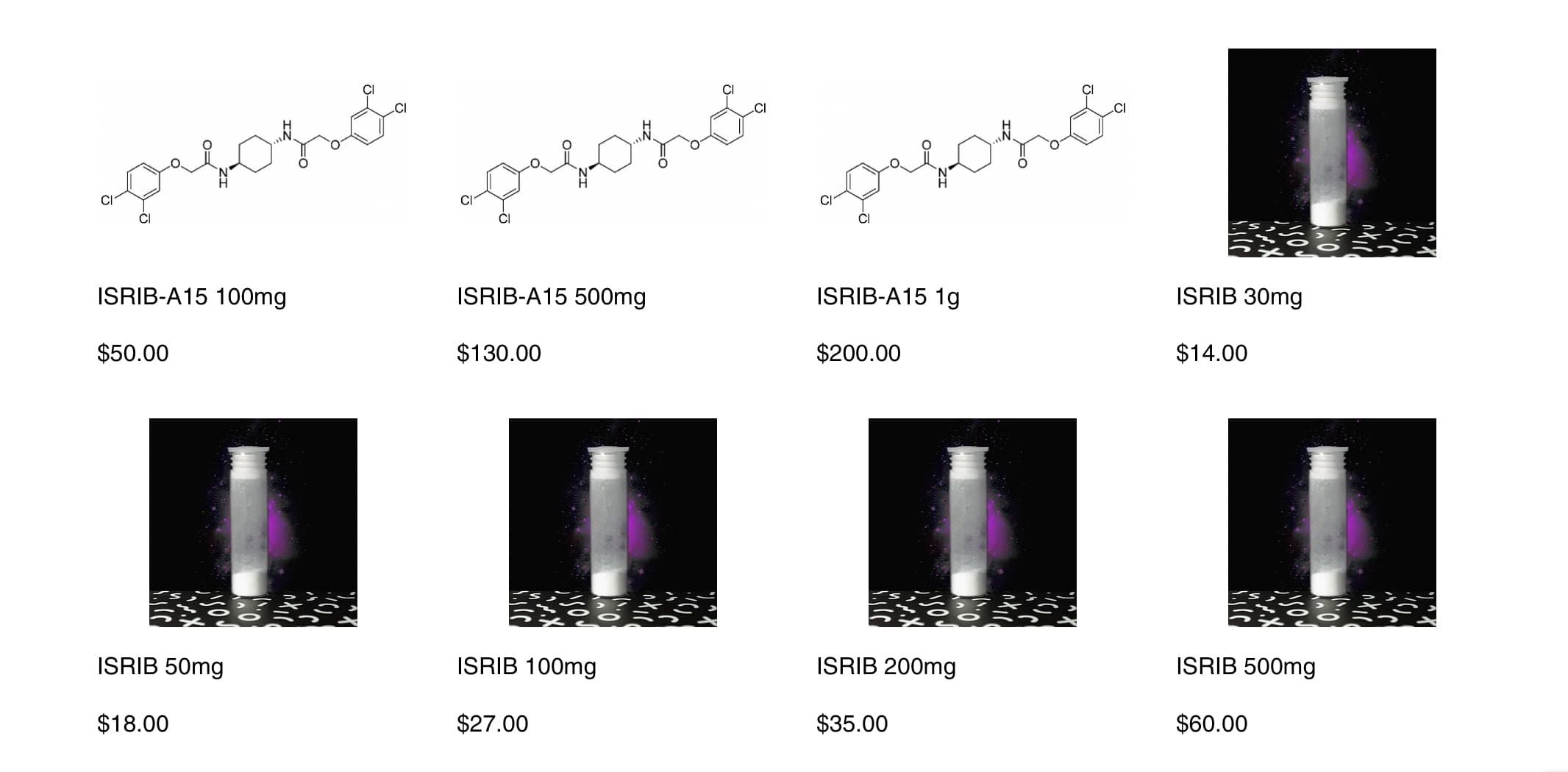
JC said most the group buyers dropped out / stopped responding for ABBV-CLS-7262 so currently unsure if it’ll happen.
It would be a very compelling purchase if it included a lab test validation of the compound from an independent 3rd party US-based analytical chemistry lab. Just add the cost to the total cost of the group purchase.
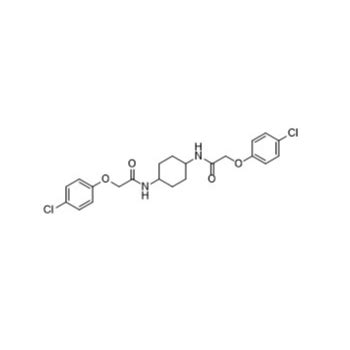
This is probably the basic structure of ISRIB.
Several variants are synthesized too; named ISRIB-1… ISRIB-18 or having more obscure naming.
This substance seems to be available through several sources like:
ReMind ISRIB - Купить ноотроп ИСРИБ (10 мг × 10 капсул) в Москве | FitHerb 10 caps for some 3600 Rubble
others including regular chemical companies to be found when you follow the links under the images from searching with ISRIB wiki.
modifications that can (or probably already have been) made include adding extra atoms like chlorine, fluoride or methyl on the para-chloro-phenol groups, replacing of the C=O groups or moving them towards the phenolic oxygen and/or changes on the middle ring and a lot more tricks that synthetic chemistry people would consider.
Some of the resulting substances may be inactive in humans, other may be even more active than the basic molecule in the structure formula above(post 23) and other substances may be highly toxic or just do the reverse of what we want (prolong healthy lifespan).
Disappointing news… this drug failed in ALS (though that doesn’t mean it necessarily doesn’t work as an ISRIB modulator I suspect). But it seems the drug won’t be pursued as a commercial product…
US-based biotechnology company Denali Therapeutics has reported that a Phase II/III trial of DNL343 for amyotrophic lateral sclerosis (ALS) failed to meet its primary endpoint.
The primary analysis included 186 subjects who received the therapy and 139 subjects who were given a placebo. The drug was investigated as part of the HEALEY ALS Platform trial.
Its primary endpoints were to assess changes in disease severity over time using the ALS Functional Rating Scale-Revised, as well as survival rates up to week 24.
The trial’s secondary endpoints assessed respiratory function and muscle strength. These showed no significant difference between the therapy and placebo groups at the 24-week mark.
Denali reported that the therapy was safe and well-tolerated among subjects.
The company anticipates further analyses later this year, which will include neurofilament light (NfL) and other fluid biomarkers, as well as data from pre-specified subgroups and extended outcomes from the active treatment extension period.
So two ISRIB analogs failed for ALS? That doesn’t sound good, but maybe still worth digging into if it means that dosage and safety info become publicly available, along with the substances themselves.
Yes, Good ideas…
Calico/Abbvie and Denali’s twin failures for phase 2 ALS using an eIF2B activator, key component of the integrated stress response (ISR) pathway
AbbVie, Calico and Denali Therapeutics’ hopes of treating amyotrophic lateral sclerosis (ALS) by targeting eIF2B have all taken a hit. Massachusetts General Hospital found neither AbbVie and Calico’s fosigotifator nor Denali’s DNL343 significantly slowed disease progression, causing misses on their respective primary endpoints.
Mass General tested fosigotifator and DNL343 in separate regimens of its HEALEY ALS Platform trial. Both molecules are designed to activate the protein complex eIF2B, protecting neurons by preventing further aggregation of the TDP-43 protein. Mass General evaluated the molecules—and other candidates—in parallel in a perpetual phase 2/3 study that will run until safe and effective ALS therapies are found.
The top-line results dent hopes that fosigotifator and DNL343 can meet the unmet need in ALS. Neither drug candidate significantly slowed ALS progression compared to placebo after 24 weeks or improved outcomes on key secondary endpoints that looked at muscle strength and respiratory function.
Both readouts contain some causes for optimism, though. The trial generated exploratory evidence that a high dose of fosigotifator may slow declines in muscle strength and revealed a potential effect on lung function. The hopes for DNL343 rest on upcoming assessments of prespecified subgroups, biomarker results and longer-term follow-up data.
Evercore analyst Mike DiFiore questioned whether six months is enough time to see an effect on disease progression at an event with a Denali executive last month. Alex Schuth, chief operating and financial officer at Denali, said DiFiore made “a very fair point,” adding that “frankly we would have probably wanted to treat longer than six months,” but patients and Mass General favored the shorter time frame.
Read the full story:
Bromantane, a mild stimulant, improves working memory, increases motivation, and is a nice mood elevator.
I just noticed these guys followed my Rapamycin News X / Twitter account… another potential source for ISRIB:
it would be nice to have some independent lab verification of their product quality and purity, contaminants, etc. But, perhaps in a short term test via ingestion, less of an issue? Please post if you try them…