Yes. Risk avoidance happens in people at a prodromal stage, before official PD diagnosis. So if they are avoiding surgery it might select for those who have the surgery to be more non-PD people, making it seem like surgery is protective. Reverse causation. Meanwhile PD patients on dopa drugs escalate risk taking, gambling etc. - so if we could find stats that show PD people on dopa drugs having more plastic surgery than PD people not on those drugs, we might establish if this hypothesis has evidence for it.
They’re definitely higher income / net worth than average which correlates positively with intelligence so it’s all tangled up.
I moved the above posts here as they were unrelated to hypoxia.
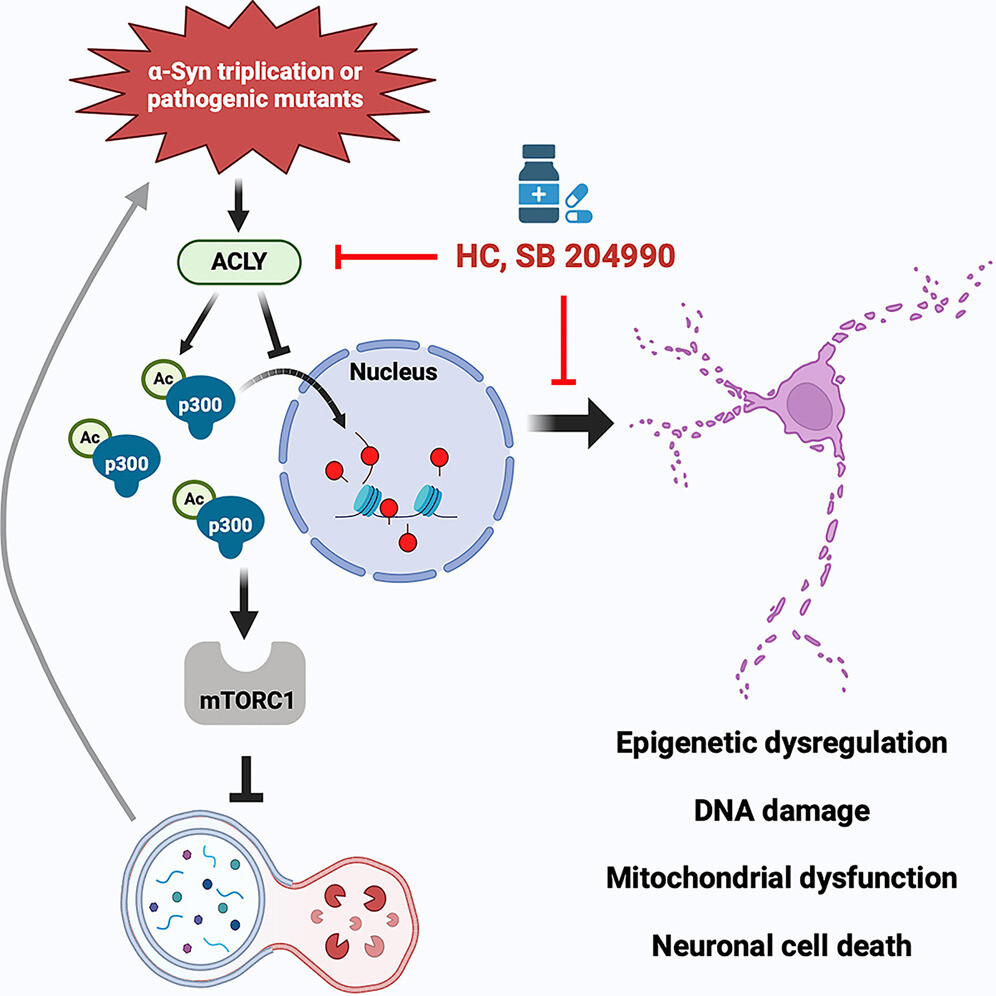
α-Syn mutations activate ACLY, which enhances cytoplasmic p300 activity
Cytoplasmic p300 hyperactivation stimulates mTORC1 to inhibit autophagy
ACLY inhibition restores autophagy and reduces α-Syn aggregates in neuronal models
ACLY inhibitors rescue pathology in both zebrafish and mouse α-synucleinopathy models
Triplications and certain point mutations in the SNCA gene, encoding alpha-synuclein (α-Syn), cause Parkinson’s disease (PD). Here, we demonstrate that the PD-causing A53T α-Syn mutation and elevated α-Syn expression perturb acetyl-coenzyme A (CoA) and p300 biology in human neurons and in the CNS of zebrafish and mice. This dysregulation is mediated by activation of ATP-citrate lyase (ACLY), a key enzyme that generates acetyl-CoA in the cytoplasm, via two mechanisms. First, ACLY activity increases acetyl-CoA levels, which activate p300. Second, ACLY activation increases LKB1 acetylation, which inhibits AMPK, leading to increased cytoplasmic and decreased nuclear p300. This lowers histone acetylation and increases acetylation of cytoplasmic p300 substrates, like raptor, which causes mechanistic target of rapamycin complex 1 (mTORC1) hyperactivation, thereby impairing autophagy. ACLY inhibitors rescue pathological phenotypes in PD neurons, organoids, zebrafish, and mouse models, suggesting that this pathway is a core feature of α-Syn toxicity and that ACLY may be a suitable therapeutic target.
PD is strongly linked to epigenetic dysregulation, particularly in alterations of histone modification. Previous studies have reported an alteration in the acetylation of histone H3, which correlated with both Lewy body stage and substantia nigra pigmentation scores.47,48 In addition, genome-wide analyses showed dysregulation of H3K27 acetylation in the prefrontal cortex of PD brains.49 Another study showed H3K4me3 was increased in PD bulk substantia nigra.50 Pathogenic A53T α-Syn inhibits H3 acetylation by binding directly to histones11 or through interaction with the transcriptional adapter 2-alpha (TADA2a).22 Despite the growing evidence for the significance of epigenetic changes in PD brains, the mechanisms by which these changes occur and how they induce pathological phenotypes remain unclear. Our data suggest that such alterations may, in part, be due to decreased nuclear p300 levels/activities.
Our data suggest that since A53T α-Syn causes ACLY activation, which hyperactivates mTORC1 and causes autophagy inhibition, there is the potential for a positive feedback loop, as α-Syn is itself an autophagy substrate that will accumulate further after autophagy is compromised. It is likely that other pathways, like proteasomal degradation, can buffer the deleterious effects of autophagy defects in A53T α-Syn-expressing cells or cells with SNCA triplication. However, if such buffering pathways, or autophagy itself, are further compromised, then such a feedback loop could be unleashed. We speculate that one of the reasons why many neurodegenerative diseases only manifest in later age is because such feedback processes are kept in check for decades until additional processes like aging, which compromise brain autophagy,51 overwhelm the homeostatic balances.
What do you think @John_Hemming?
What this seems to say is that there is a cycle which is sensible really.
PD is strongly linked to epigenetic dysregulation , particularly in alterations of histone modification.
So that is my hypothesis.
The real issue for acetylation is the availability of the substrate to create acetyl-CoA which is our good friend 2-hydroxy-1,2,3-propanetricarboxylate. * C6H5O7-3
Something coming down the pipeline. @adssx
It’s quite interesting to me, I’d give it a go ![]()
And I happen to know a guy…
ASHA-091 is designed to specifically inhibit DRP1 activation, a regulatory protein with a role in the fragmentation of mitochondria.
“A key feature of most neurodegenerative disease … is the hyper-fragmentation of mitochondria. This promotes neuroinflammation, neuronal dysfunction, and ultimately neurodegeneration,” the company reports on a therapy webpage. ASHA-091 is a “first-in-class highly specific inhibitor of mitochondrial fragmentation that restores normal cellular function.”
This is a fun story about a woman that can smell parkinson’s. Her ability was turned into a test that predicts the disease much earlier than has been done before:
Given that Parkinson’s is most likely caused by long term exposure to industrial toxins (eg herbicides and insecticides) taking supplements that boost Glutathione and other detoxification pathways might be a good way to stop progression of Parkinson. This is supported indirectly by Parkinson onset being preceded by 10 years or more of reduced Glutathione levels. Gluthathione levels decrease with age.
Grey hair is the most visible symptom of Glutathione depletion in old age, and results from accumulation of hydrogen peroxide in the blood, which bleaches hair follicles.
Supplementing directly with glutathione does not work well, since it is broken down by digestion, so works no better than supplementing with NAC (a source of cysteine, the critical amino acid used for glutathione synthesis in the body). However, the enzymes required for glutathione synthesis decreases with age, so relying on NAC supplements requires increasing the dosage as you age and eventually requires very high doses of up to 9 g/day, which has the drawback of producing high levels of toxic Hydrogen Sulfide in the gut, which might not be a good idea with Parkinson’s since toxins that cause Parkinson’s are believed to travel from the gut to the brain via the Vagus nerve (bypassing the BBB).
Using IV glutathione doesn’t work either, since the glutathione just circulates in the blood and cannot be absorbed by neurons in the brain (though liver cells are able to absorb some glutathione from the blood).
Two alternate ways to raise Glutathione in the brain that avoid the toxic side effects of NAC:
-
Intranasal Glutathione, which may be able to bypass the BBB, though the experimental results so far are disappointing : https://www.michaeljfox.org/news/ask-md-glutathione-and-parkinsons, possibly because neurons, like most cells, have trouble absorbing Glutathione.
-
Oral supplementation with Gamma-Glutamyl-Cysteine, a precursor that does NOT break down in the gut and is easily absorbed by all cells (and converted to Glutathione inside cells without depending on the enzyme that decreases with old age). Unfortunately the only source is the commercial product Glyteine from Continual-G which costs $1.5 per 400mg dose and you need at least 2 doses per day. I use 4 doses per day, since each dose is only effective for around 3-4 hours : I don’t have Parkinson’s but I do have constant brain fog and trouble with memory unless I use this supplement…
There may be additional detoxification pathways that may need to be strengthened, using supplements like Milk Thistle or Green Tea extract , but the evidence for benefits for Parkinson’s so far is not good.
Interesting. I don’t know if glutathione depletion is the mechanism, but there does appear to be some potential association with gray hair.
Preclinical signs of Parkinson’s disease: A possible association of Parkinson’s disease with skin and hair features
https://www.sciencedirect.com/science/article/abs/pii/S0306987719301707
“We hypothesize that earlier age at onset of hair greying, greater tendency to sunburn, difficulty tanning and dysregulation of sebum production are more common among PD patients due to genetically determined lower constitutive amounts of melaninand accumulation of α-synuclein in the skin, which leads to disrupted synthesis of peripheral melaninand dysregulated sebum secretion.”
dramatic rethink of Parkinson’s offers new hope for treatment
Mounting evidence suggests there might be two separate types of the world’s fastest-growing neurological condition. Can this fresh understanding lead to much-needed new treatments?
PER BORGHAMMER’S “aha” moment came nearly 20 years ago. The neuroscientist was reading a paper from researchers who were examining whether REM sleep behaviour disorder (RBD), a condition that causes people to act out their dreams and is often found in people who later develop Parkinson’s disease, could be an early form of the neurological condition.
Rather than starting with the brain, however, the team instead looked for nerve cell loss in the heart. Though Parkinson’s is historically associated with nerve cell depletion in the brain, it also affects neurons in the heart that manage autonomic functions such as heart rate and blood pressure. And, says Borghammer, “In all of these patients, the heart is invisible; it is gone.”
Sign up to our Health newsletter
Receive a weekly dose of discovery in your inbox.
Sign up to newsletter
➔
Not literally, of course. But in these people, the neurons that produce the neurotransmitter norepinephrine, which helps control heart rate, were so depleted that their hearts didn’t show up on scans using radioactive tracers. This kind of neuron loss is associated with Parkinson’s, but at the time, none of the people had been diagnosed with the disease and their brain scans seemed normal.
What struck Borghammer was that Parkinson’s didn’t seem to follow the same trajectory in everyone it affected: RBD strongly predicts Parkinson’s, but not everyone with Parkinson’s experiences RBD.
“I realised that Parkinson’s must be at least two types,” says Borghammer – when neuron loss starts outside the brain, eventually working its way in, and when neuron loss is largely restricted to the brain from the beginning. By 2019, Borghammer, at Aarhus University in Denmark, had gathered enough evidence to formally propose his theory of “brain-first” and “body-first” Parkinson’s.
Now, with 14 published studies and more on the way, the idea is starting to gather steam. And if Borghammer is right, reframing the disease as existing in two discrete forms could dramatically transform how we treat or even prevent it.
Read more The brain has its own microbiome. Here’s what it means for your health
“I think that we are getting to the heart of what causes Parkinson’s disease,” says Timothy Greenamyre, a neurologist at the University of Pittsburgh in Pennsylvania. “That will be a huge home run.”
Getting to grips with the causes of Parkinson’s disease can’t come soon enough. Cases are skyrocketing, with a recent study estimating that by 2050, 25.2 million people will be living with Parkinson’s disease worldwide, more than double the nearly 12 million people with it in 2021. Some researchers are even calling it a “pandemic” . Meanwhile, the search for more effective treatments, not to mention a cure, has been littered with disappointments.
Parkinson’s symptoms – tremors, unstable gait, muscular rigidity – were documented as far back as 600 BC. But it wasn’t until the 19th century that the condition became known as Parkinson’s, after London physician James Parkinson described six people’s “involuntary tremulous motion” in his 1817 An Essay on the Shaking Palsy. Parkinson’s was then rare, and it would take 100 years before the brain structures involved were identified.
Losing dopamine
We now know that Parkinson’s is associated with the loss of nerve cells in parts of the brain that help control movement, such as the substantia nigra. Some of these neurons produce the neurotransmitter dopamine, and the reduction in dopamine disrupts the normal signalling pathways that control motor function, leading to the “tremulous motion” Parkinson observed.
This neuronal die-off appears to be caused by the proliferation of a misfolded form of the protein alpha-synuclein. Alpha-synuclein is found throughout our bodies and plays a critical role in controlling the release of neurotransmitters, including dopamine, at synapses, the junctions between neurons where communication happens. But for proteins to function within cells correctly, they need to assume the right shape. When alpha-synuclein misfolds, it can form clumps called Lewy bodies inside neurons. The Lewy bodies slowly kill the neurons, whether by disrupting signalling, puncturing cells or accumulating in the mitochondria, inhibiting cells’ ability to produce energy.
What causes alpha-synuclein to misfold is still unclear, but the irregular proteins seem to then spread the disease from cell to cell. “One of the mechanisms that cells have to defend themselves against bad proteins is to get rid of it by kicking it out of the cell with exosomes – a small balloon of bad material,” says Borghammer. “And then the neighbour is stupid enough to import it, and then you have kick-started the process in the next cell.”
Trying to understand why and how alpha-synuclein goes wrong, researchers began to search for the places in the body where the misfolding could originate. In the 1990s, neuroanatomist Heiko Braak at Goethe University Frankfurt in Germany observed that the proliferation of the Lewy body clumps resembled “a falling row of dominoes”. This led him to suspect that the disease might originate outside the central nervous system and somehow find its way in. In 2003, Braak proposed that some kind of pathogen could trigger local inflammation in a network of nerve cells within the gut called the enteric nervous system and initiate the corruption of alpha-synuclein. Neurons in the vagus nerve, a conduit that connects the gut and brain, would then carry the misfolded protein to the vulnerable brain regions.
Braak’s hypothesis has gained ground in the years since. However, critics note that it doesn’t describe the development of Parkinson’s in all cases. In a small but pivotal 2020 study, Borghammer, Aarhus University PhD student Jacob Horsager and their colleagues assessed 37 people with Parkinson’s, of whom 13 also had RBD, as well as 22 people with only RBD. The team showed that, on average, people with RBD had more neuron loss in the heart and gut than those with only Parkinson’s, hinting that the disease originated there before making its way to the brain.
But crucially, the team also found that those without RBD “lose the dopamine system first but have more normal hearts and guts”, says Borghammer, implying that for them, the disease started in the brain.
Borghammer realised that, though Braak might have been correct in thinking that neuronal degradation starts “body-first” in some people with Parkinson’s, that didn’t describe everyone. There are other people for whom the dopamine-producing structures in the brain are affected from the start, “brain-first”. “It is completely two separate categories with no overlap,” says Borghammer.
Brain-first, body-first
Borghammer’s post-mortem analyses of people who died with Parkinson’s offered yet more evidence that the disease followed at least two trajectories: some people had misfolded alpha-synuclein only in the centre of their brain, supporting the brain-first idea, but for others, it was found only at the bottom of the brainstem, as if it had just reached the brain from somewhere else. “When you’ve got several hundred brains [showing this], it starts to get pretty convincing,” says John Hardy, a neurologist at University College London.
Read more We’re finally learning how perimenopause profoundly changes the brain
Borghammer’s team isn’t alone in pursuing this idea. Married neurologists Valina and Ted Dawson at Johns Hopkins University in Maryland have tested the body-first theory by injecting misfolded alpha-synuclein into mice’s guts. “It just seemed like a reasonable experiment to do, to formally test the hypothesis,” says Ted Dawson.
One month later, the misfolded protein was in the mice’s brains, killing off their dopamine-producing neurons and inducing the onset of symptoms such as movement difficulties and loss of smell. “They got the whole spectrum of disease,” says Valina Dawson.
Crucially, this didn’t occur for mice that had their vagus nerve cut shortly after the injections. “I think that the data is very persuasive,” says Dario Alessi, who researches Parkinson’s genetic pathways at the University of Dundee, UK.
Such research cannot ethically be done in humans, but scientists can study people who had their vagus nerve cut as a last-resort treatment for peptic ulcer disease. In one study, those who had the nerve cut at the junction between the oesophagus and the stomach – the “trunk” of the nerve tree that communicates with digestive organs – were 15 per cent less likely to develop Parkinson’s 20 years later than people in the general population who hadn’t had the procedure. In a separate study, Borghammer and his team uncovered more evidence for a gut connection. They analysed gut tissue samples taken from 57 people up to 20 years before they were diagnosed with Parkinson’s disease and found misfolded alpha-synuclein in more than half of them, at significantly higher levels than in people who never developed the disease.
Though much of the research focus in body-first Parkinson’s has been on the gut, some scientists have gone looking for and found misfolded alpha-synuclein in other places in the bodies of people who don’t have neuron loss in the brain, including the appendix and the nasal cavity. “I think that it is plausible that an initiating event in a Parkinson’s disease cascade can occur in the periphery and then move centrally,” says Alastair Noyce, a neurologist at Queen Mary University of London.
The two subtypes also align with how differently the condition can manifest in people. “We are confronted with a very broad spectrum of what we call Parkinson’s disease that can be very differentially expressed in different patients,” says Filip Scheperjans, a neurologist at Helsinki University Hospital in Finland.
For example, people with signs of body-first Parkinson’s – exhibiting misfolded alpha-synuclein in peripheral tissues outside of the brain – are more likely to experience disruption to autonomic systems. In such people, RBD, unexplained drops in blood pressure, urinary dysfunction and constipation can occur years before their movement is affected. “When you see them in the street, you wouldn’t know that this is a sick person,” says Borghammer. “And in 70 per cent of these cases, when you do a dopamine scan, it is normal, [but] sooner or later it becomes abnormal.”
For brain-first Parkinson’s disease, movement-related symptoms dominate from the start. “These are the people who are more likely to have tremor,” says Camille Carroll at the University of Plymouth, UK.
New paths to treatment
Knowing that Parkinson’s might actually be two different types of disease can offer new pathways to treating it. “The point is: why the hell is there a brain-[first] and body-first type?” says Borghammer. “If there are some differences – molecular differences, genetic differences, cellular differences – these might constitute treatment targets, but we have no idea, because nobody has studied Parkinson’s disease in this framework.”
At the moment, says Borghammer, only a handful of medical centres around the world have the scanning equipment needed to differentiate between body-first or brain-first forms of the disease. However, future studies could divide trial participants into these two groups, so drugs are tested on those who have the best shot of benefiting from them This could be particularly important when targeting the gut microbiome, which can be dramatically altered in people with Parkinson’s disease.
Several teams are already working on this. In a study published in 2020, neurologist Haydeh Payami at the University of Alabama at Birmingham examined the gut microbiomes of 490 people with Parkinson’s and 234 people without the condition. She found that 30 per cent of the species of gut microorganisms in people with Parkinson’s were either abnormally elevated or depleted, compared with those without the condition. One species that was elevated was Escherichia coli; some kinds of E. coli have been found to induce alpha-synuclein misfolding in the gut. Some bacteria can also stimulate inflammation, which could damage dopamine-producing neurons in the gut, says Scheperjans.
But these findings throw up the age-old dilemma of correlation versus causation. Is the gut microbiome intrinsically different in people who will go on to develop Parkinson’s or do symptoms such as constipation lead to changes? To investigate this question, a team led by microbiologist Sarkis Mazmanian at the California Institute of Technology transplanted faecal samples from people with Parkinson’s disease into germ-free mice bred to overexpress the normal alpha-synuclein protein, seeding the rodents with bacteria from the patients’ guts. Within six weeks, the mice developed signs of impaired movement, including being unable to perform mouse-specific motor function tests as well as before. “That is a nice step in the direction of causation,” says Carroll.
That raised the tantalising idea that the reverse procedure – transplanting healthy bacteria into the guts of people with Parkinson’s – could treat symptoms. The prospect has shown promise in animal studies and in at least one 2024 human trial that found “mild, but long-lasting beneficial effects” on motor symptoms in people with early-stage Parkinson’s.
Other research, however, has shown mixed results. In July 2024, Scheperjans and his colleagues gave 45 people with mild to moderate Parkinson’s either a faecal microbiome transplant (FMT) from a healthy donor or a placebo infusion. Six months later, there was no difference in movement-related symptoms between the two groups. However, those who had a transplant went on to need a lower dose of levodopa, a drug that helps replace the lost dopamine, than those in the placebo group. The transplant may have improved their body’s ability to use levodopa, so they required a smaller dose even if their symptoms progressed as much as those who had a placebo, says Scheperjans.
Researchers aren’t yet done investigating the link between the gut microbiome and Parkinson’s symptoms: two more FMT studies are under way, as well as a trial testing the antibiotic rifaximin’s effects on symptoms via its action on the gut microbiome. And though the July 2024 trial wasn’t quite the bullseye researchers might have hoped for, it could nevertheless lend further support to the idea that Parkinson’s exists in two types. Because the trial didn’t separate participants into subtypes, it’s unclear whether the treatment could be beneficial solely for individuals whose Parkinson’s originates in the gut, for example. “It could be that there are some mechanisms that we try to attack with new treatments, [but] they only work for one subtype,” says Horsager, who was not involved in the trial.
And that, say researchers, is an incredibly important step in the direction of more effective treatment. “We need to be able to give tailored treatments to subgroups of patients that really benefit from them,” says Borghammer. “How do we get there? We get there by subtyping.”
For more on what’s new in science
Follow New Scientist
+
But more than that, says Horsager, “It has revolutionised our understanding of the disease, if it is correct. We have to start thinking about the disease in whole new way.”
Parkinson’s is the fastest-growing neurodegenerative condition in the world. The question is: why? Initial rises in disease rates were attributed to increased life expectancy – diagnoses generally occur among people aged 60 or older and “as more people get old, there’ll be more people with Parkinson’s”, says Dario Alessi at the University of Dundee, UK. But that can’t be the whole answer. Rates of Parkinson’s are rising faster than would be expected even if people are living longer, says Filip Scheperjans at Helsinki University Hospital in Finland.
Many point the finger at pesticides, which have been the subject of dozens of Parkinson’s-related studies over the past 40 years. They enter cells and damage mitochondria, which provide cells with energy, says Alessi. “You get to that level where the body can’t compensate anymore and you start getting symptoms.” Much of this research is observational and cannot prove cause and effect, but the sheer volume of evidence makes the idea increasingly convincing. “It is very robust, very consistent across studies,” says Alastair Noyce at Queen Mary University of London. Studies of agricultural workers also show that greater pesticide exposure is linked to greater likelihood of diagnosis. “If you sprayed more [pesticides] and were protected less, that seemed to increase your risk even more,” says Lee Neilson at Oregon Health & Science University.
Air pollution is also increasingly under scrutiny. Traffic exhaust fumes release particles known as PM2.5. These measure just 2.5 micrometres across and contain even smaller particles that can cross the blood-brain barrier, the membrane that keeps harmful substances in the blood out of the brain, triggering inflammation that may damage dopamine-producing neurons. In a recent study, people who had the greatest PM2.5 exposure, determined by their home address, were 23 per cent more likely to have a Parkinson’s diagnosis than those with the lowest exposure. However, the scientific consensus isn’t unanimous – defining PM2.5 exposure is difficult, as is quantifying an individual’s exposure over their lifetime.
Psilocybin Shows Promise for Parkinson’s Mood and Motor Symptoms - Neuroscience News
Another molecule that possibly can benefit.
Waiting for Bicep to chime in on this one!
On schedule. ![]()
In conclusion, DENR inhibitors represent a promising class of therapeutic agents with diverse applications in cancer, viral infections, neurodegenerative diseases, and immune modulation.
Another exciting application of DENR inhibitors is in the field of neurodegenerative diseases. Conditions such as Alzheimer’s and Parkinson’s disease are associated with dysregulated protein synthesis and aggregation of misfolded proteins. By modulating the ribosome recycling process, DENR inhibitors could help restore protein homeostasis and alleviate the toxic effects of protein aggregates in neuronal cells.
A bit more on Parkinson’s and Magic Mushrooms. Seems promising.
Link: ‘Magic mushrooms’ may offer major relief for Parkinson’s patients, study shows | Fox News
Legal to prescribe in Oregon in 2021 and California in 2023.
California Gov. Gavin Newsom (D) has signed a bill that would allow doctors to immediately start prescribing certain currently illicit drugs like psilocybin and MDMA if they’re federally rescheduled, and he also approved separate legislation to amend THC variance testing requirements for marijuana.Oct 2, 2023.
Apologies if this has been posted before, but I think this one is very interesting. There used to be the idea that before dopamine agonist use, PD patients were risk averse, and after were the opposite. But what if that’s wrong? This is where mendelian randomization comes in.
**Risky behaviors and Parkinson disease
A mendelian randomization study**
Unhealthy Behaviours and Risk of Parkinson’s Disease: A Mendelian Randomisation Study
And the ny times in the same study https://www.nytimes.com/2025/05/07/well/eat/ultraprocessed-foods-linked-to-early-symptoms-of-parkinsons.html?unlocked_article_code=1.Fk8.Y-0o.EMuBTxLznDCk&smid=url-share
Rapamycin exerts neuroprotective effects by inhibiting FKBP12 instead of mTORC1 in the mouse model of Parkinson’s disease
Parkinson’s disease (PD), characterized by the selective loss of nigral dopaminergic neurons, is a common neurodegenerative disorder for which effective disease-modifying therapies remain unavailable. Rapamycin, a clinical immunosuppressant used for decades, has demonstrated neuroprotective effects in various animal models of neurological diseases, including PD. These effects are believed to be mediated through the inhibition of mammalian target of rapamycin (mTOR) complex 1 (mTORC1) signaling, with rapamycin binding to FKBP12. However, recent studies have suggested that mTOR activation can be neuroprotective in degenerating dopaminergic neurons, presenting a paradox to the neuroprotective mechanism of rapamycin via mTORC1 inhibition. In this study, we showed that mTORC1 signaling was inactivated in nigral dopaminergic neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD. Notably, the optimal neuroprotective dose of rapamycin did not inhibit mTORC1 signaling nor restore autophagy defects in nigral dopaminergic neurons of MPTP-treated male C57BL/6 mice. Furthermore, acute Raptor knockout in dopaminergic neurons, which abolishes mTORC1 activity, did not diminish rapamycin’s neuroprotective effects, suggesting that its protection is independent of mTORC1 inhibition. Importantly, rapamycin is also a potent inhibitor of FKBP12, a peptidyl-prolyl cis-trans isomerase highly expressed in the brain. Selective knockdown of FKBP12 in nigral dopaminergic neurons confers neuroprotective effects comparable to that of rapamycin, with no synergism observed when the two are combined. Collectively, our results indicate that rapamycin exerts neuroprotective effects in parkinsonian mice through inhibition of FKBP12 rather than mTORC1 signaling. These findings suggest that FKBP12 may serve as a novel target for disease-modifying therapies in PD.
As a note, it’s an MPTP model of PD so considered very low value (essentially useless).