Seems my SBP has now come down from the 120-140 range to 105-120 with the Telmisartan 40 mg. I like the new range better.

For all the Ted Cruz out there.
You really need a good home unit and a reliable process to get a handle on blood pressure.
- Check to be sure your monitor is validated here. And be sure to buy one on the list if you are buying a home unit.
- Be sure to follow best practices for monitoring.
- Always take bp at the same time of day. Morning, a half hour or so after waking is best in my experience.
- Follow instructions carefully. Position of cuff, arm at heart level, etc.
- Relax for a few minutes, take a measure, wait a minute, take another, etc. Average of 3 readings.
- Track your average over time, and pay attention to trends
-
Best option in US for a monitor is the OMRON bronze BP5150. If you are in the market for one now, as of Nov 15, it is available for $35 on amazon. It has bluetooth and the app is actually pretty good.
-
Target 90-120 for SBP and 60-80 for DBP. Pulse pressure should be close to 40 (difference between the SBP and DBP)
-
I personally aim for 105/65. In my extensive research, it is achievable by most healthly people, is right in the range of healthy according to guidelines, and is unlikely to cause issues such as orthostatic hypotension, or lightheadedness.
-
Using 40mg telmisartan is a great option to get your bp a little lower. That is my first choice option. I am currently at 115/70 average. Hoping 40-80 mg telmisartan will help me achieve my goal.
Aktiia is as good as any other home BP monitor but it measure continuously 24/7 for like 5-7 days without needing another charge.
Unfortunately, it’s nowhere as good, I posted a few reviews previously: Aktiia is especially bad a night. During the day, it’s okay, especially if you take the weekly average. But individual measures can be quite off.
Can you explain more in detail with why you think it’s okay during the day, and what you mean with individual measures being quite off?
It might be worth to have a manual BP machine then and take BP every now and then to make sure.
This is what the academic papers testing Aktiia concluded. See:
- Angiotensin II receptor blocker (ARB) experiences? - #14 by adssx
- Angiotensin II receptor blocker (ARB) experiences? - #91 by adssx
- Angiotensin II receptor blocker (ARB) experiences? - #153 by adssx
- Angiotensin II receptor blocker (ARB) experiences? - #164 by adssx
Some were independent while some were sponsored and/or conducted by Aktiia.
I was wondering what you mean with okay – compared to what? What’s great?
And what you mean with individual measures being off.
Just click on these four links and read the papers. They compared Aktiia to home cuff measurements and/or 24h ABPM. My tl;dr: at night: Aktiia bad, during the day: Aktiia okay-ish. Weekly average = Aktiia perfect.
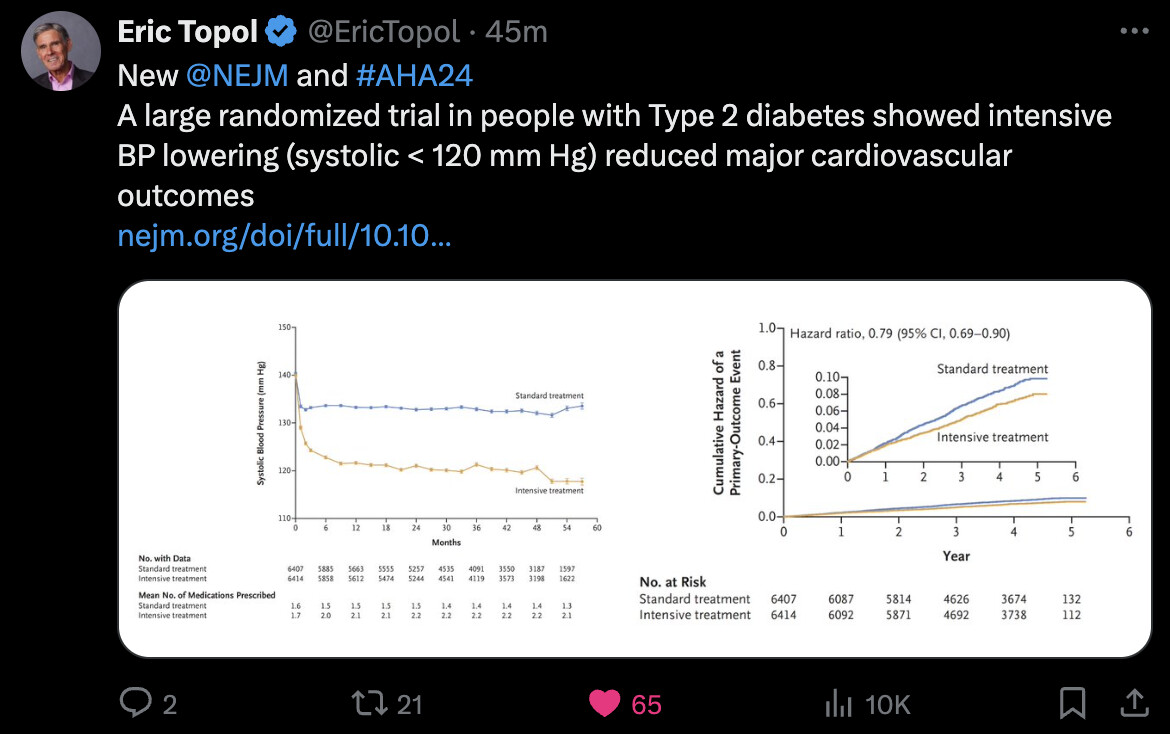
Intensive Blood-Pressure Control in Patients with Type 2 Diabetes 2024
Does this extend to people without diabetes? What about all-cause deaths? Which antihypertensive did they use?
Note that they compared a 120 mm Hg target to a 140 one and not to 130 mm Hg.
I think it is sad that there seems to be a developing tendency to leave the intervention out of the abstract.
I think in this case they probably didn’t direct the intervention but told doctors to use the same drugs as usual with target X or Y. That’s usually what these trials do. What I’d like to see is: you take people with BP at 130 mmHg and you give them telmisartan + amlodipine (or placebo) to go to 120 mmHg and you look at the MACE and ACM after 5y.
I’ve been using Aktiia but it has been disappointing from the beginning, skipping many measurements during the day, while being better at measures during the night. However, the values at night appeared pretty low. I don’t know if the comparison with other devices at night is homogeneous, only if the device is unobtrusive and doesn’t alter the BP (for example, by awakening the user).
I’ve had software problems a few months ago and didn’t even call assistance. I just bought a good Omron home device.
A&D UA-651BLE there’s 2 of these on ebay for 11.99 shipped. Bluetooth plus the app will log to Apple Health or the android equivalent. This is listed on the validatebp.org site, btw.
The update pertains to three types of Ramipril 2.5 mg capsules:
- 90 count (NDC 68180-589-09, Lots G326781, exp. date September 30, 2025, GA04468, exp. date May 31, 2025 )
- 100 count (NDC 68180-589-01, Lots G326763, exp. date September 30, 2025, GA03041, exp. date March 31, 2026, GA03725, exp. date April 30, 2026, GA04402, exp. date May 31, 2026)
- 500 count (NDC 68180-589-02, Lots G326782, exp. date September 30, 2025, GA04462, exp. date May 31, 2026)
However, a letter written by The California State Board of Pharmacy indicates that an additional 8 lots of Ramipril Capsules USP 5 mg and 13 lots of Ramipril Capsules USP 10 mg were also recalled to the retail level.
According to the FDA’s notice, the products were found to have used manufacturing techniques that deviated from Current Good Manufacturing Practice (cGMP). The health authority notes that an “active pharmaceutical ingredient was sourced from an unapproved vendor.” It was not immediately clear from the recall notice which ingredient was considered compromised, but the California Board calls it a “key starting material.”
Bring Your Blood Pressure Down! - Medical Frontiers
This is a very interesting video, with a lot of data:
Some recent info on Telmisartan: @DrFraser
Telmisartan 40 mg is working well. Down from the 130-140 range to 110-120. I will be going to 80 mg to get into the 100-110 range.
Excellent result, well done.
I’m not a doctor but my understanding is that it can often be better to go to lower dose dual or even tri therapy rather than raising the dose of monotherapy for BP control. Have you considered adding another agent like Nebivolol for example?
I want to go to Telmisartan at 80 mg because there are pleiotropic effects on HBA1C at 80 mg. It should also lower SBP another couple points.