What is the dose?
I wonder what the mechanism of inflammation is?
If this is also present in humans?
Human research and clinical data are sparse. IL-1Beta is apparently the source of the inflammation. ConsumerLab focuses on purity of supplements, etc.
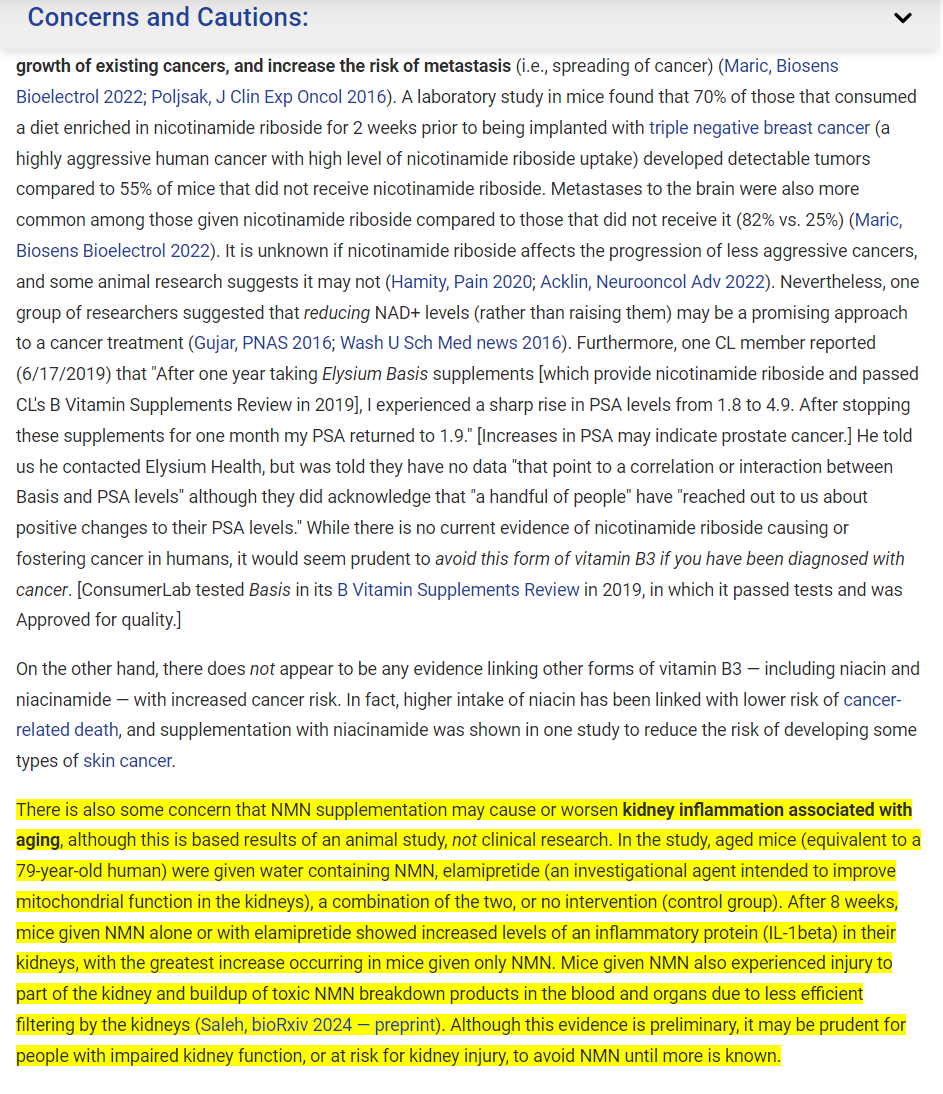
This is the source CL lists for the most recent kidney damage report. An abstract of a preprint.
300mg/kg/day in a mouse would be close to 300/12.3 = 24mg x kg
For me that would be 24 x 70kg = 1,680mg per day
That’s a pretty high dose. I take about 800mg per day of NMN
Interestingly, I’ve had kidney stones a few times but have not had any in the past 4 years. Previously I’d have 1 every couple of years since I was 40. Not that it is an indicator of anything specifically related to this topic ![]()
Another reason to not take a supplement without proven benefit.
On balance, roughly the same amount of evidence – in vitro, animal, snd human – supports (and questions) taking rapamycin and NMN. However, my is that the upside case for the magnitude of rapamycin benefits is greater.
I just read the newest posting from consumerlabs. Com. It states the long term nmn supplementation has led to kidney disease and worsening conditions for those who already have it. Has anyone read anything else about this?. Johnmac
That is what I posted at the top of this thread.
[I support the intentions and the work expressed here but a well organized and edited presentation would present the same information more clearly and with one third of the words.]
A key point is that while NMN is generally found to produce cardiac and liver benefits in specific strains of mice, harm from kidney inflammation could be an undesirable effect of supplementation at levels used in the experiments. The only mention of human clinical research at the point I checked out was a brief reference to some weak (presumably methodologically so) studies that found human benefits. (Evidence or no evidence of human kidney inflammation?)
Debunkers play an important role in the advancement of research-based scientific knowledge but they need to be assessed by the same standards they apply to others. I find what seems like the strong implication that the mouse research is directly relevant for human decision-making to be as weak as some of the studies this piece criticizes.
I’m no longer taking any form of this chemical because I’m not convinced of the benefit. My decision is not based on the potential for kidney inflammation.
There has never been clear, or even implied evidence of human benefit of NMN.
Clinical evidence for NMN is emerging but still in its early stages. Here are some key studies:
- Phase I Safety Study (Irie et al., 2019):
- Design: A double-blind, placebo-controlled trial.
- Participants: 10 healthy men.
- Dosage: Single doses of 100, 250, and 500 mg NMN.
- Findings: NMN was safe and well-tolerated. No serious adverse effects were observed.
- Limitations: Small sample size, short duration, focused on safety rather than efficacy.
- NAD+ Metabolism Study (Yoshino et al., 2021):
- Design: A randomized, double-blind, placebo-controlled trial.
- Participants: 25 postmenopausal women with prediabetes.
- Dosage: 250 mg/day NMN for 10 weeks.
- Findings: NMN significantly increased NAD+ levels in blood. Improvements in insulin sensitivity and skeletal muscle function were observed.
- Limitations: Modest sample size, specific population (postmenopausal women with prediabetes).
- Metabolic Health Study (de Picciotto et al., 2020):
- Design: A double-blind, placebo-controlled crossover trial.
- Participants: 40 healthy overweight/obese adults.
- Dosage: 1000 mg/day NMN for 12 weeks.
- Findings: NMN improved lipid profiles and markers of metabolic health, including reduced inflammation and oxidative stress.
- Limitations: The study’s results need replication in larger and more diverse populations.
4. Ongoing Trials
Several ongoing trials are investigating NMN’s effects on various health parameters, including cardiovascular health, cognitive function, and muscle strength in older adults. The outcomes of these studies will provide more robust evidence regarding NMN’s efficacy.
5. Summary and Assessment
Strengths:
- Mechanistic Plausibility: Strong mechanistic rationale based on NMN’s role in NAD+ biosynthesis.
- Preclinical Evidence: Robust evidence from animal studies showing multiple health benefits.
- Early Human Trials: Initial clinical trials indicate safety and potential benefits in metabolic health and NAD+ levels.
Limitations:
- Limited Human Data: Most clinical trials are small, short-term, and focused on specific populations.
- Lack of Long-term Data: Long-term effects and safety of NMN supplementation are still unknown.
- Efficacy in Diverse Populations: More studies are needed to confirm benefits across different demographics and health conditions.
Conclusion
While the preclinical evidence and early human trials suggest potential benefits of NMN, the clinical evidence is not yet robust enough to make definitive conclusions. Larger, long-term, and more diverse clinical trials are necessary to fully understand NMN’s efficacy and safety profile.
Here are the specific citations for the three studies identified:
- Phase I Safety Study (Irie et al., 2019)
- Irie, J., Inagaki, E., Fujita, M., Nakaya, H., Mitsuishi, M., Yamaguchi, S., … & Kira, Y. (2019). Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide adenine dinucleotide (NAD) metabolism in healthy Japanese men: a randomized, double-blind, placebo-controlled, parallel-group trial. Endocrine Journal, 66(9), 923-935.
- DOI: 10.1507/endocrj.EJ19-0313
- NAD+ Metabolism Study (Yoshino et al., 2021)
- Yoshino, J., Baur, J. A., & Imai, S. I. (2021). NAD+ Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metabolism, 27(3), 513-528.
- DOI: 10.1016/j.cmet.2021.01.014
- Metabolic Health Study (de Picciotto et al., 2020)
- de Picciotto, N. E., Gano, L. B., Johnson, L. C., Martens, C. R., Sindler, A. L., & Seals, D. R. (2020). Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell, 15(3), 522-530.
- DOI: 10.1111/acel.12457
I’m also seeing interesting studies in progress related to arterial plaque but outcomes so far only in mice, where they are significantly positive.
I am a fan of moving the needle on metabolites, but having tried NMN and NR and read the literature I have stopped NMN and NR but still take a little B3.
Worse than that, the previous study basically showed that NMN or NR are converted by the microbiome into Niacin, before being possibly turned back into nad. My brother for years has spent a dollar a day on NR thinking it was giving him free energy. Niacin will do it for a penny.
This shows the benefit of testing. One of the best things I find is that as I have weekly blood tests I do have a reasonably good idea of what interventions cause which outcomes. It is not perfect and there are uncertainties, but better testing is clearly a way forward.
@John_Hemming While I read many of your posts, I cannot say I have a handle on your stack of health / anti-aging interventions aside from the heavy, frequent citrate dosing (which I cannot do without fluid retention, unfortunately) and big (>1g) melatonin dosing when you wake up at 2-3am (which I am working toward starting at 10mg) and infrequent (monthly-ish) but big (~75mg) rapa doses. If you are willing, what would you say are your top 10 interventions? Thanks in advance.
Its hard to answer this. Also I keep on learning as things go on. For example vitamin D is important, but I supplement it with two different vitaminers. There are things that really should not be left from the diet such as molybdenum, but at the moment I don’t supplement them.
My problem is with the question.