I’ll put Mike’s videos in this thread when I see them and think they are interesting…
Introduction to Biological Age and Blood Testing
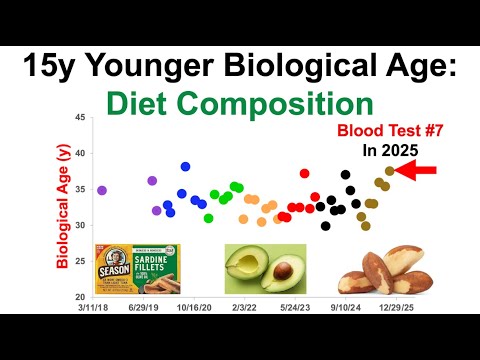
- The video discusses the results of a blood test conducted in 2025, indicating a biological age that is significantly younger than the chronological age.
- The speaker questions whether the biological age clock, specifically the pheno age, has any blind spots and introduces the nine biomarkers that contribute to this assessment.
- These biomarkers include measures related to liver health, kidney function, metabolic health, immune response, and red blood cells, but notably exclude cardiovascular disease biomarkers.
- The absence of cardiovascular disease-related biomarkers is emphasized as significant since cardiovascular disease ranks among the top five causes of death.
- A younger biological age is correlated with a lower risk of death from cardiovascular disease, suggesting the importance of tracking additional biomarkers related to cardiovascular health.
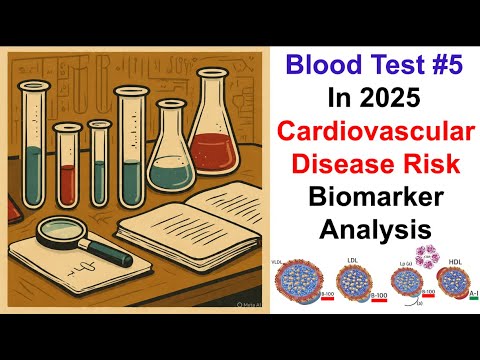
Cardiovascular Disease Risk Biomarkers
- The speaker plans to delve into cardiovascular disease risk biomarkers, beginning with details from the blood test report.
- Tests are ordered through ultalabtest.com, allowing for personalized selection of tests, followed by a blood draw at Quest Diagnostics.
- Results from a test conducted on July 22nd were received just four days later, highlighting the efficiency of the process.
Lipid Panel Results - HDL
- The speaker reviews HDL (high-density lipoprotein) levels from the lipid panel, noting a result of 76 milligrams per deciliter, which is above the reference range of 40 milligrams per deciliter.
- While the result is good, the speaker refers to a more stringent optimal range of 50 to 69 milligrams per deciliter based on extensive studies involving millions of participants.
- HDL levels tend to decline with age, and the speaker has created a Patreon tier to share data on optimal biomarker ranges as they relate to aging.
- The speaker examines HDL data spanning 20 years, revealing fluctuations and a general decline prior to 2015, which coincided with the beginning of a more rigorous dietary tracking approach.
- Since adopting a detailed dietary tracking method, the speaker has managed to increase HDL levels significantly, with a recent average of 58 milligrams per deciliter.
APOA1 Levels and Their Significance
- APOA1, a component of HDL, is discussed as an important biomarker for cardiovascular disease risk, with the speaker presenting a current test result of 166 milligrams per deciliter.
- The reference range for APOA1 is anything over 115 milligrams per deciliter, indicating that the speaker’s result is satisfactory.
- The speaker notes that the reference range has not been updated since a 2004 study, despite newer data being available.
- The optimal range for APOA1, based on recent studies, is identified as 150 to 180 milligrams per deciliter, suggesting that the speaker’s levels are adequate but could be improved.
- The speaker’s average APOA1 levels over six tests are 146 milligrams per deciliter, which is slightly below the optimal range but shows potential for improvement.
Triglyceride Levels and Assessment
- The triglyceride level from the latest test is reported at 62 milligrams per deciliter, which is well below the reference threshold of 150 milligrams per deciliter.
- However, the speaker aims for an optimal triglyceride level of less than 90 milligrams per deciliter, as studies indicate this range is associated with lower all-cause mortality risk.
- In a study involving 4.5 million participants, the lowest coronary heart disease risk was associated with triglyceride levels below 45 milligrams per deciliter, which is the speaker’s long-term goal.
- Over 20 years and 71 tests, the speaker has noted a trend of decreasing triglycerides, although recent data shows an upward trend that the speaker hopes to reverse through increased cardio activity.
- The speaker reflects on dietary changes, including a brief period of veganism, and the impact of a low-fat diet on triglyceride levels.
LDL Levels and Long-Term Trends
- The LDL (low-density lipoprotein) level for the recent test is noted at 86 milligrams per deciliter, which is below the reference range of 100 milligrams per deciliter.
- The speaker discusses optimal LDL levels, citing studies that associate the lowest coronary heart disease risk with levels ranging from 65 to 120 milligrams per deciliter.
- The speaker acknowledges the complexity of LDL trends, which typically follow an inverse U-shape across the lifespan, peaking in midlife before declining in older age.
- Analysis of LDL data from 72 tests over 20 years reveals fluctuations that may relate to dietary changes made since 2015.
- The speaker aims to stabilize LDL levels while avoiding age-related increases or decreases, with a long-term average of 82.5 milligrams per deciliter.
Lipoprotein A and Cardiovascular Risk
- The speaker’s lipoprotein A level is reported at 91 nanomolar, which exceeds the reference range of 75 nanomolar, raising concerns about cardiovascular risk.
- Optimal levels for lipoprotein A, associated with the lowest coronary artery disease risk, are considered to be below 48 nanomolar.
- Historical data from 45 tests over 20 years shows a concerning average of 106 nanomolar for the first decade, indicating poor lipoprotein A levels.
- The speaker cites a significant improvement in lipoprotein A levels over the past 10 years, achieving an average of 90 nanomolar through dietary changes.
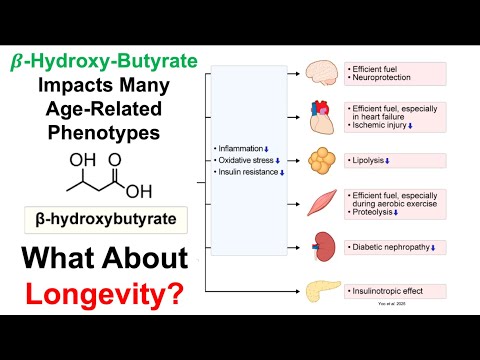
- The speaker notes the importance of high-sensitivity C-reactive protein (hsCRP) levels in modifying cardiovascular risk associated with lipoprotein A.
High-Sensitivity C-Reactive Protein and Its Impact
- The speaker’s hsCRP level is reported as less than 0.2 milligrams per liter, indicating a low level of inflammation, which is favorable for cardiovascular health.
- The speaker has consistently maintained hsCRP levels below the detection limit for 26 tests, suggesting effective management of inflammation over the years.
- Despite a higher lipoprotein A level, low hsCRP may mitigate cardiovascular disease risk, particularly given the speaker’s family history of cardiovascular issues.
- The relationship between lipoprotein A and cardiovascular disease risk remains complex, especially when factoring in hsCRP levels.
APO B Levels and Future Monitoring
- APO B, a protein associated with various lipoproteins, is discussed, with a current level of 71 milligrams per deciliter, which is below the reference range of 90 milligrams per deciliter.
- The speaker aims for an optimal APO B level of less than 70 milligrams per deciliter, which is associated with lower cardiovascular disease and all-cause mortality risk.
- Over six tests, the speaker has achieved an average APO B level of 67 milligrams per deciliter, indicating that he is within the optimal range.
- The speaker plans to continue monitoring APO B to gather more data and assess its relevance compared to other lipoprotein measures.
Conclusion and Future Directions
- The video concludes with the acknowledgment that only a portion of the blood test report has been covered, encouraging viewers to seek further information available on Patreon.
- The speaker plans to share details regarding the diet, supplements, and medications that correspond to the recent test results in future videos.
- Viewers are invited to follow the speaker’s journey in biohacking aging and to access various resources, including affiliate links for testing services.
- The speaker emphasizes the importance of understanding one’s health metrics in the context of aging and cardiovascular disease prevention.
AI Summary done using this website: Krisp | Free Youtube Video Summarizer with AI