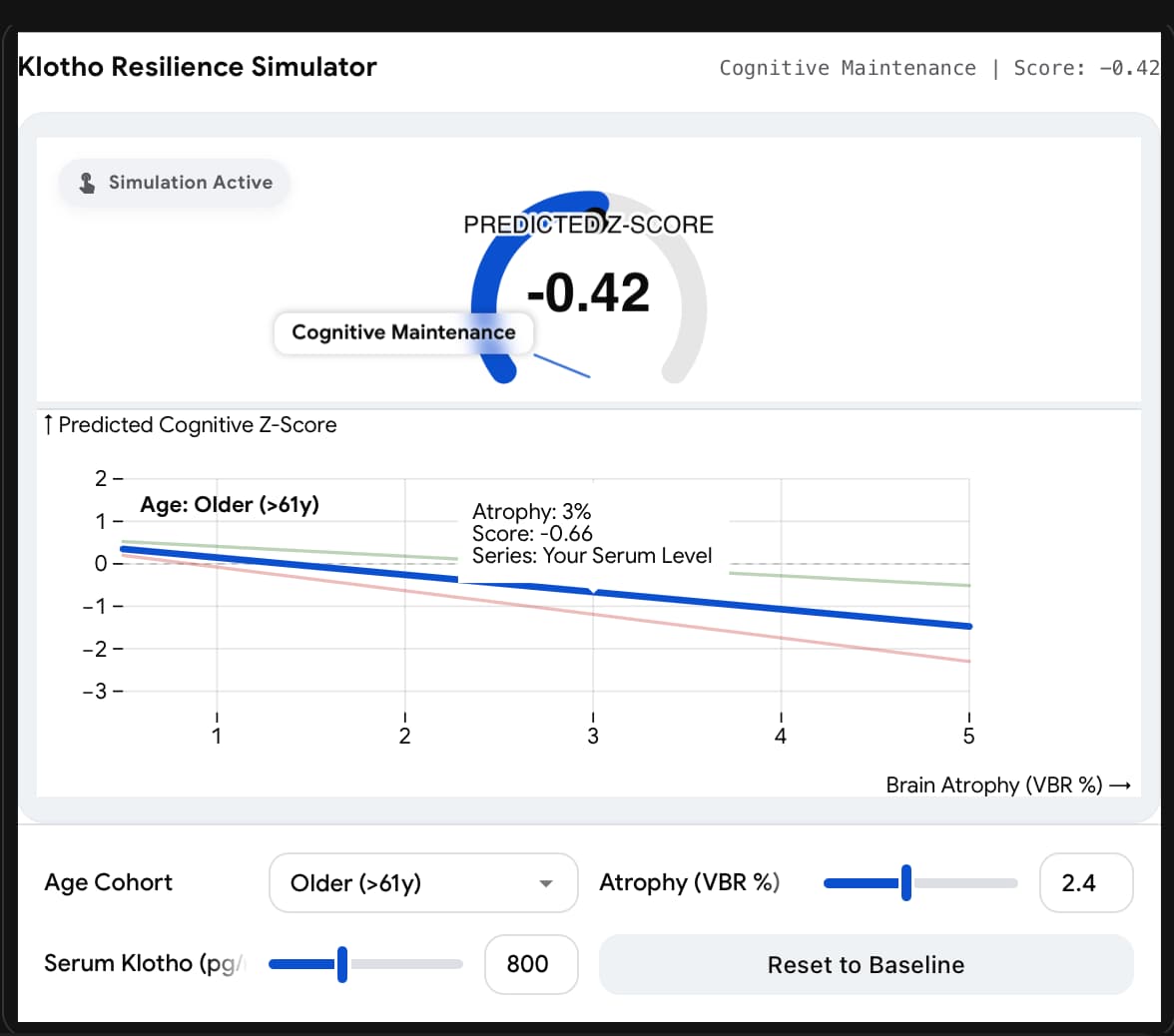
A new study reveals a critical age-dependent twist in the story of Klotho, the celebrated “longevity protein.” For years, biohackers have pursued Klotho elevation as a universal strategy for cognitive enhancement and life extension. This research, however, suggests that Klotho’s neuroprotective benefits may be strictly reserved for the elderly. In a cohort of 308 cognitively unimpaired adults, higher serum Klotho levels successfully shielded older adults (>61.6 years) from the cognitive deficits usually caused by brain atrophy (ventricular expansion). Effectively, high Klotho allowed these older brains to “function through the damage.”
Conversely, and shockingly, in younger adults (<61.6 years), higher Klotho levels were associated with worse cognitive performance. This suggests a potential case of antagonistic pleiotropy—where a mechanism beneficial in late life might be deleterious or metabolically costly in mid-life. For the longevity community, this is a massive signal: “more is better” does not apply universally to Klotho. The protein appears to act as a resilience factor against structural decay (atrophy) specifically when that decay is present, but may disrupt optimal signaling in healthier, younger brains.
Source:
- Paywalled Paper: Serum Klotho Levels, Brain Structure, and Cognitive Performance
- Institution: University of Wisconsin-Madison, Wisconsin Alzheimer Disease Research Center, USA.
- Journal: JAMA Neurology. February 2, 2026
- Impact Evaluation: The impact score of this journal is 21.3 (2024 JIF), this is an Elite impact journal (ranked ~3rd in Clinical Neurology).
Part 2: The Biohacker Analysis
Study Design Specifications
- Type: Human Cross-Sectional Observation (Correlational).
-
Subjects: 308 middle-aged and older adults (Mean age: 61.3 ± 6.5 years).
- Demographics: 80% Female, 96% White, Highly Educated (~16.4 years).
- Group Stratification: Younger (≤61.6 years, n=154) vs. Older (>61.6 years, n=154).
-
Key Biomarkers:
- Serum α-Klotho: Measured via ELISA.
- Brain Structure: Ventricle-Brain Ratio (VBR) via MRI (proxy for cerebral atrophy).
- Cognition: Composite scores for global cognition, executive function, delayed recall, and immediate learning.
Lifespan Analysis
- Mouse Lifespan Data: N/A – Human Clinical Study.
- Note: The paper references animal data where Klotho extension (via genetic overexpression) increases lifespan by ~20-30%, but no new lifespan data was generated in this specific document.
Mechanistic Deep Dive
The study highlights a “Resilience Mechanism” rather than direct structural repair.
- Structural Disconnect: High Klotho did not prevent brain atrophy (VBR); older adults with high Klotho still had atrophy. However, high Klotho uncoupled this atrophy from cognitive decline.
-
Proposed Pathways:
- Synaptic Fortification: Klotho enhances NMDA receptor function (GluN2B subunits), boosting Long-Term Potentiation (LTP) even in damaged circuits.
- Antioxidant/Anti-inflammatory: Upregulation of the Nrf2 pathway and suppression of Wnt signaling, reducing neuroinflammation that typically exacerbates structural volume loss.
- Amyloid Clearance: Potential enhancement of autophagy and blood-brain barrier transport of A$\beta$, though this study found Klotho’s benefits were independent of Amyloid/Tau status.
Novelty
- Age-Specific Antagonism: Previous dogma held that Klotho is universally good. This study provides human clinical evidence of antagonistic pleiotropy, showing that high Klotho correlates with lower executive function and memory in mid-life (age ~55).
- Resilience > Repair: This confirms Klotho acts as a “buffer.” It doesn’t stop the brain from shrinking (atrophy), but it stops the symptoms of that shrinkage from manifesting as dementia.
Critical Limitations & Biohacker Takeaways
- 1. The “White/Educated” Distortion: The sample was 96% White and highly educated (Master’s degree level average). This is a massive failure in generalizability. We cannot assume these metabolic correlations hold for other ethnicities with different baseline Klotho dynamics (e.g., African American populations often have different KL-VS variant frequencies).
- 2. Temporal Mismatch: The MRI scans and blood draws were not simultaneous—sometimes separated by over a year. While statistically adjusted, this introduces noise in the correlation between acute serum levels and chronic structural atrophy.
- 3. Reverse Causality Risk: In the younger group, “high Klotho = bad cognition” could be reverse causality. Perhaps early pathological stress causes a compensatory spike in Klotho? The cross-sectional design cannot rule this out.