Snorting doesn’t work for water insoluble??
You might be right that it is absorbed to a certain extent, but most of what I found on the absorption of water-insoluble drugs is negative. Here is one example:
https://www.sciencedirect.com/science/article/abs/pii/S0939641123000504
What is the dosing (mg) and frequency you are doing?
My dosing is inconsistent, but I have been gradually ramping up from 10 mg with 1 mL of DMSO towards 20 mg with 2 mL assuming the ratio of ISRIB to DMSO is at the higher end of 9 mg/1 mL. Most on that subreddit use doses ranging from 10-40 mg which I am guessing has something to do with the doses used in animal studies. I have seen studies as low as 0.1 mg/kg in animals which means most human doses are already at a very high level- almost to par with mice dosing.
Here is an average for animal doses of ISRIB:
At 0.25 mg/kg, there was at least one study saying it penetrated the BBB well. The challenge, though, is that it needing to be dissolved in DMSO makes it a bit rough getting down, even at 10% dilution.
This interesting reading… thanks for pointing towards this reddit group on ISRIB and biohacking:
I wonder if shelving it would work?
is isrib-a15 the trans form?
is dmso also diluted in water?
They appear to be slightly different. ISRIB-A15 has this data: CAS number:1628478-12-5
and Formula:C22H22Cl4N2O4. Trans-ISRIB has a CAS number: 1597403-47-8 and the Formula: C22H24Cl2N2O4. Neither of the forms are in development anymore, but the only difference they ever appeared to have was absorption-related.
And definitely dilute DMSO. I think I read before that even 50+ percent DMSO is technically safe for oral consumption, but some people on that subreddit were apparently using 99.9 percent DMSO undiluted—probably not the best long-term plan. It is never used in clinical settings above 10 percent as far as I can gather.
My main concern with ISRIB (or more potent variations like ISRIB-a15, if for some reason they’re easier to get) is lack of ADME information for humans. @Luminary , how do you monitor your dosing to know you’re achieving your goals? Is there some sort of identifiable sign of overdose?
I found this good mice-to-human dose calculator: Practical PK Calculators
This gives the range of doses for ISRIB that were mentioned here: https://www.alzdiscovery.org/uploads/cognitive_vitality_media/ISRIB.pdf
Mice | Human (For 70 kg human)
.1 mg/kg = 0.569 mg (unsuccessful at this dose)
.25 mg/kg/day = 1.423 mg
2.5 mg/kg/day = 14.23 mg
5 mg/kg/day = 28.46 mg
There really is no good long-term safety or toxicity info. I did read that 5 mg/kg/day (28.46 mg) showed increased mortality and was lowered to 2.5 mg/kg/day. Personally, I have taken it only a few times at 10-20 mg, but I guess this would indicate it is probably best to be more conservative in dosing, as even 0.25 mg/kg produced some positive benefits in studies.
As for positive benefits, I only based my perception of that on the feeling of being in good cognitive shape the last few days. I can not give any solid evidence that those feelings are real.
ISRIB is quite interesting, pretty cool to see a few people here experimenting with it. Been thinking of giving it a go ![]()
So far what I’ve seen in the studies is that it is to be used as a “reset” type intervention. 2 or 3 doses over 3 to 6 days and repeat once or twice a year.
I’ll be digging in to the available papers over the next couple of weeks.
Please keep us updated on what you are finding.
Note(!): I just would like to add that do NOT use plastic/painted measuring spoons for the DMSO. DMSO will act as a solvent for any impurities and you will absorb a ton of toxins. Glass/stainless steel measuring spoons are required if used orally. And also, the FDA has not approved any oral usage of DMSO. The 10 percent dilution for usage is practiced for experimental therapies apparently. So, be safe about all of this, whoever tries it.
I would also like to add a general word of caution here about suppliers… be careful with this stuff.
I was at the Longevity Center Rountable conference this week and there was a presentation by Dr. Jordan Shlain, who started and runs a network high-end concierge medical clinics (Private Medical) for the wealthy. He has many of the tech millionaires and billionaires in the SF Bay Area, and so he gets people who see and know Bryan Johnson and want to try things like that. But Jordan Shlain has been in business for something like 20 years, isn’t a “longevity doctor”, and operates a slightly more traditional concierge medical clinic that focuses on preventative medicine, but also treats traditional ailments and disease processes people encounter.
But - since he serves the tech wealthy, many of his clients are interested in “longevity therapies” and trying them with other doctors in the SF Bay Area, or more broadly.
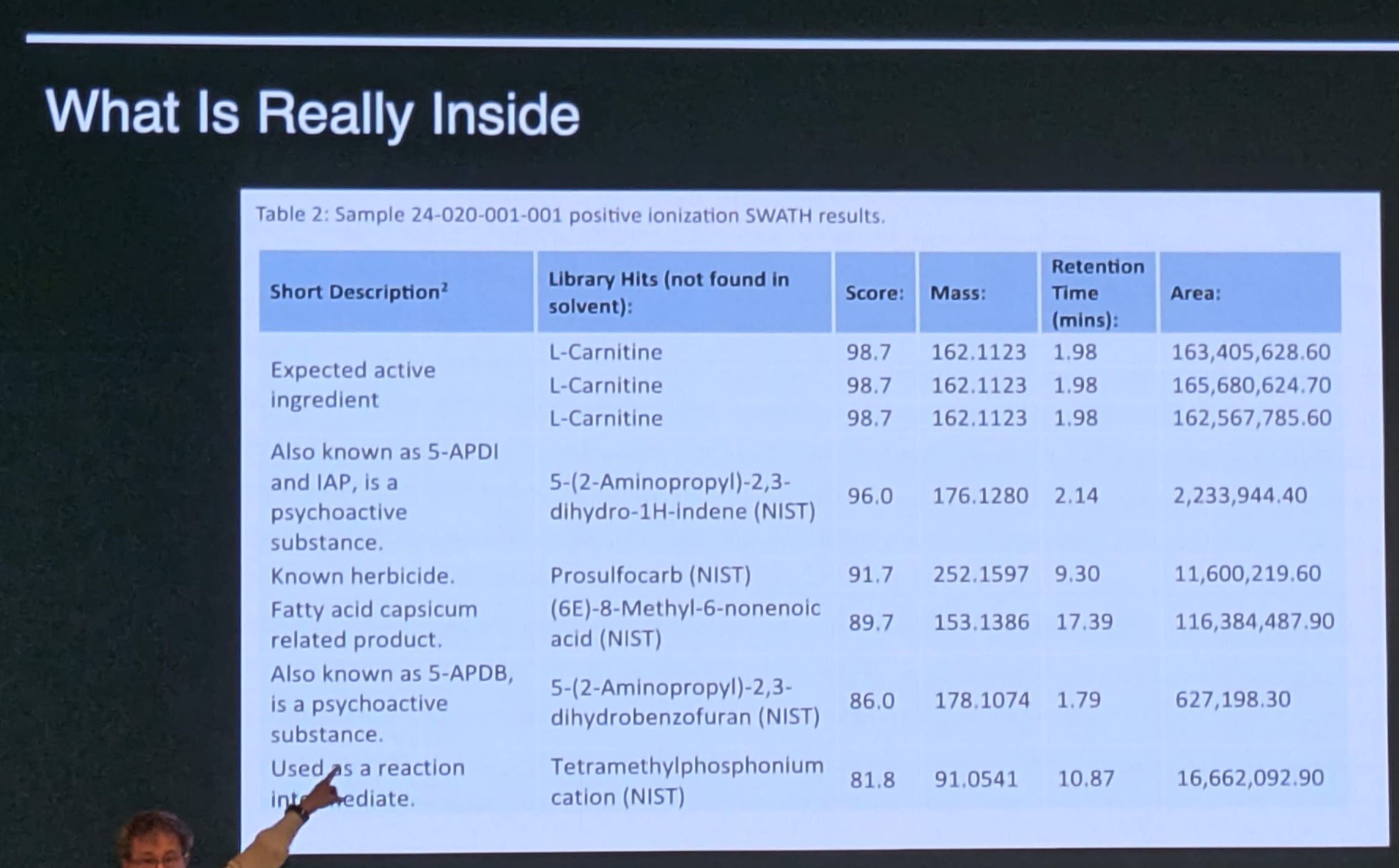
One of his clients came in after talking with a “longevity doctor” who had recommended the guy do injections of L-Carnitine. The client wanted Dr. Shlain to buy the compound for him (I guess it was only sold to doctors) but Dr. Shlain had never heard of this procedure before or supplier, and he was skeptical. So, he told the guy the first thing they should do is order the product/liquid and get a trusted lab to do an analysis on it to validate what the product actually was.
When they did the lab analysis on the product they found all sorts of chemicals that were not listed on the product’s label. Moreover, some of these chemicals were psychoactive compounds that would have a significant impact on how the person would perceive how he felt (i.e. he’d “feel great”, but it was from these additional compounds, not necessarily the L-Carnitine). So it seems vendors may be adding compounds to trick customers into thinking they are getting a great result from the product, when in fact they are getting an effect from things that are added in). So, you’d be getting more than you bargained for, and might get deceived into continually using a product because of its perceived “beneficial effects” which are not real.
Key Take Away: Be skeptical, and do validation lab testing with a good 3rd party lab of any unusual products, prior to use. See below for this example:
The Product:
What was actually in the product:
By the way, @AlexKChen when I saw you at the Longevity Summit you mentioned you had a new supplier for ISRIB. Is it the one you mentioned earlier in this thread, or a completely new one, and can you please post it if its completely new.
Faulty Mitochondria Trigger Insulin Dysfunction in Pancreatic Cells, but ISRIB Restores Blood Sugar Control in Mice
Key Takeaways:
- Faulty mitochondria in insulin-producing cells trigger a stress response that disrupts blood sugar control, per a University of Michigan study.
- An experimental drug, ISRIB (Integrated Stress Response Inhibitor, ISRIB), reversed insulin defects in mice by silencing mitochondrial distress signals.
- The same mechanism was seen in liver and fat cells, suggesting a systemic link to type 2 diabetes.

Mitochondria
Affecting hundreds of millions of people worldwide, type 2 diabetes is often associated with problems in insulin production and utilization. A team of researchers from the University of Michigan has uncovered a new mechanism connecting mitochondria to dysfunction in insulin-producing cells.
In a study published in the Journal of Translational Medicine, researchers have unveiled a novel therapeutic approach that could significantly mitigate the effects of septic cardiomyopathy, a severe condition characterized by heart dysfunction due to systemic infection. This innovation hinges on the modulation of the integrated stress response, specifically targeting the ATF4-DDIT4/TXNIP pathway, which has been implicated in mitochondrial dysfunction and ferroptosis—the process of regulated cell death associated with iron metabolism and oxidative stress.
Septic cardiomyopathy, a complication commonly associated with sepsis, remains a major challenge in critical care. Patients suffering from this condition often experience significant complications, leading to increased morbidity and mortality. The complexity of sepsis-induced heart failure has left researchers grappling with a plethora of questions regarding its pathophysiology and, more importantly, effective treatment strategies. This latest research provides hope by identifying a pathway that may be crucial in restoring cardiac function during sepsis.
At the heart of the new therapeutic strategy is ISRIB, a small molecule that has demonstrated potential in enhancing the efficacy of the integrated stress response (ISR). The ISR acts as a cellular response network to various stressors, including those induced by inflammation and infection. This study systematically investigates how targeting the ATF4-DDIT4/TXNIP axis can attenuate the detrimental effects of mitochondrial dysfunction in cardiomyocytes, potentially reversing the impacts of septic cardiomyopathy.
Targeting ATF4-DDIT4/TXNIP induced mitochondrial dysfunction and ferroptosis: ISRIB as novel therapy for septic cardiomyopathy.
J Transl Med 23, 938 (2025).
At least the high exploratory dose seems to slow down the muscle strength loss progression by 30+% in 24 week trial. Regular dose failed to do much for als or muscle strength.
Here’s a non paywall article.
Some GPT5 summaries on the clinical trials and new status of this development effort:
Calico Life Sciences is not directly testing ISRIB, but rather a related molecule called fosigotifator (also known as ABBV-CLS-7262), which functions as an eIF2B activator to modulate the integrated stress response (ISR)—the same cellular pathway targeted by ISRIB. This compound is currently being evaluated in clinical trials for an ultra-rare neurological condition:
Clinical Trial Indication
Vanishing White Matter (VWM) disease
- Fosigotifator is being investigated for the treatment of Vanishing White Matter (VWM), a progressive leukoencephalopathy caused by mutations in any of the five subunits of eIF2B. These mutations impair the assembly or activity of the eIF2B complex, leading to chronic activation of the ISR and degeneration of the brain’s white matter.
- In preclinical mouse models of VWM, fosigotifator has demonstrated the ability to decrease the aberrant ISR in the brain and spinal cord, improving motor coordination and movement outcomes.
(Calico)
Trial Details
- Phase 1b/2 clinical trial is underway, evaluating the safety, tolerability, pharmacokinetics, pharmacodynamics, and exploratory efficacy of fosigotifator in individuals diagnosed with VWM.
- This trial marks the first-ever administration of an eIF2B activator in humans for VWM.
(Calico)
Broader Context & Rationale
- ISRIB, an experimental molecule first discovered in UCSF labs, similarly targets ISR by acting on eIF2B—and has shown remarkable cognitive and neuronal benefits in animal models (e.g., reversing trauma- or age-related cognitive decline).
- Calico licensed ISRIB with plans to explore it for broader neurodegenerative and cognitive dysfunction indications.
(Longevity Technology, Fight Aging!, EL PAÍS English) - However, for the initial human trials, Calico has chosen fosigotifator—likely due to better drug-like properties, oral bioavailability, or other pharmacologic considerations—rather than directly using ISRIB.
(rapamycin.news, Calico)
Summary Table
| Molecule | Target Mechanism | Clinical Indication Being Tested |
|---|---|---|
| ISRIB | eIF2B modulator – inhibits the integrated stress response (ISR) | Licensed by Calico; preclinical use only |
| Fosigotifator (ABBV-CLS-7262) | eIF2B activator—restores function in mutated eIF2B complexes | Active Phase 1b/2 trial in Vanishing White Matter disease (Calico) |
Takeaway: Calico is advancing an ISR-targeting strategy in humans—but their current clinical candidate is fosigotifator, not ISRIB, with the targeted indication being Vanishing White Matter disease.
Let me know if you’d like more details on fosigotifator’s molecular mechanism, trial design, or how this approach compares with ISRIB’s preclinical profile.
=================================
Here’s what the latest information reveals about fosigotifator (Calico/AbbVie’s eIF2B activator) and the status of ISRIB-related development programs:
Fosigotifator: Trial Outcomes & Ongoing Development
ALS HEALEY Platform Trial
- Primary endpoint missed: In the HEALEY ALS platform trial, fosigotifator failed to slow disease progression compared to placebo. Key secondary measures—ALSFRS-R, respiratory function, and quality-of-life—also showed no significant benefit at the primary dose.(Calico, Neurology live, Fierce Biotech)
- Safety profile: Well-tolerated, with similar rates of treatment-emergent adverse events compared to placebo.(Medthority)
- Exploratory signals: At a higher exploratory dose, investigators noted some positive trends in muscle strength and possibly respiratory function, prompting calls for further exploration.(Neurology live, Fierce Biotech)
Status of Other Indications (VWM, MDD, ALS, etc.)
- Active pipeline entries: AbbVie’s public pipeline listings still show fosigotifator (ABBV-CLS-7262) as being actively investigated for ALS, Vanishing White Matter (VWM) disease, and Major Depressive Disorder (MDD). The status indicates ongoing or early-phase development.(Pharmaceutical Research & Development, Wikipedia)
- VWM trials: No recent updates have emerged indicating termination; the VWM Phase 1b/2 trial appears ongoing or paused pending further evaluation.
ISRIB Development: Any Human Advancement?
- No clinical trials yet for ISRIB: There is no evidence that Calico or AbbVie have initiated clinical trials with ISRIB (the original eIF2B modulator discovered in academic labs). Development focus has instead been on the more drug-like compound fosigotifator.
- Pipeline status: ISRIB is not listed in AbbVie’s or Calico’s publicly available pipelines or trial registries.
Summary Snapshot
| Molecule / Program | Latest Status |
|---|---|
| Fosigotifator (ABBV-CLS-7262) | ALS trial failed primary endpoint; well-tolerated. Early signals at high dose may prompt further analysis. Still listed for VWM and MDD in pipeline. |
| Vanishing White Matter (VWM) | No evidence of trial termination; possibly ongoing Phase 1b/2. |
| Major Depressive Disorder (MDD) | Still under investigation per pipeline listings. |
| ISRIB | No active human trials or development programs reported. |
Bottom Line
- Fosigotifator hasn’t been abandoned—despite ALS trial failure, it remains in early-stage development for VWM and MDD.
- There are no active clinical efforts to advance ISRIB into humans at present—Calico/AbbVie appear to remain focused on fosigotifator as a more pharmacologically suitable ISR-targeting agent.
Would you like me to monitor clinical trial registries and press for updates on VWM or MDD, or to explore ISRIB derivatives or alternatives in preclinical development?
Yes, I would appreciate updates.