[Edit: Added missing charts + wrong description]
How they did it:
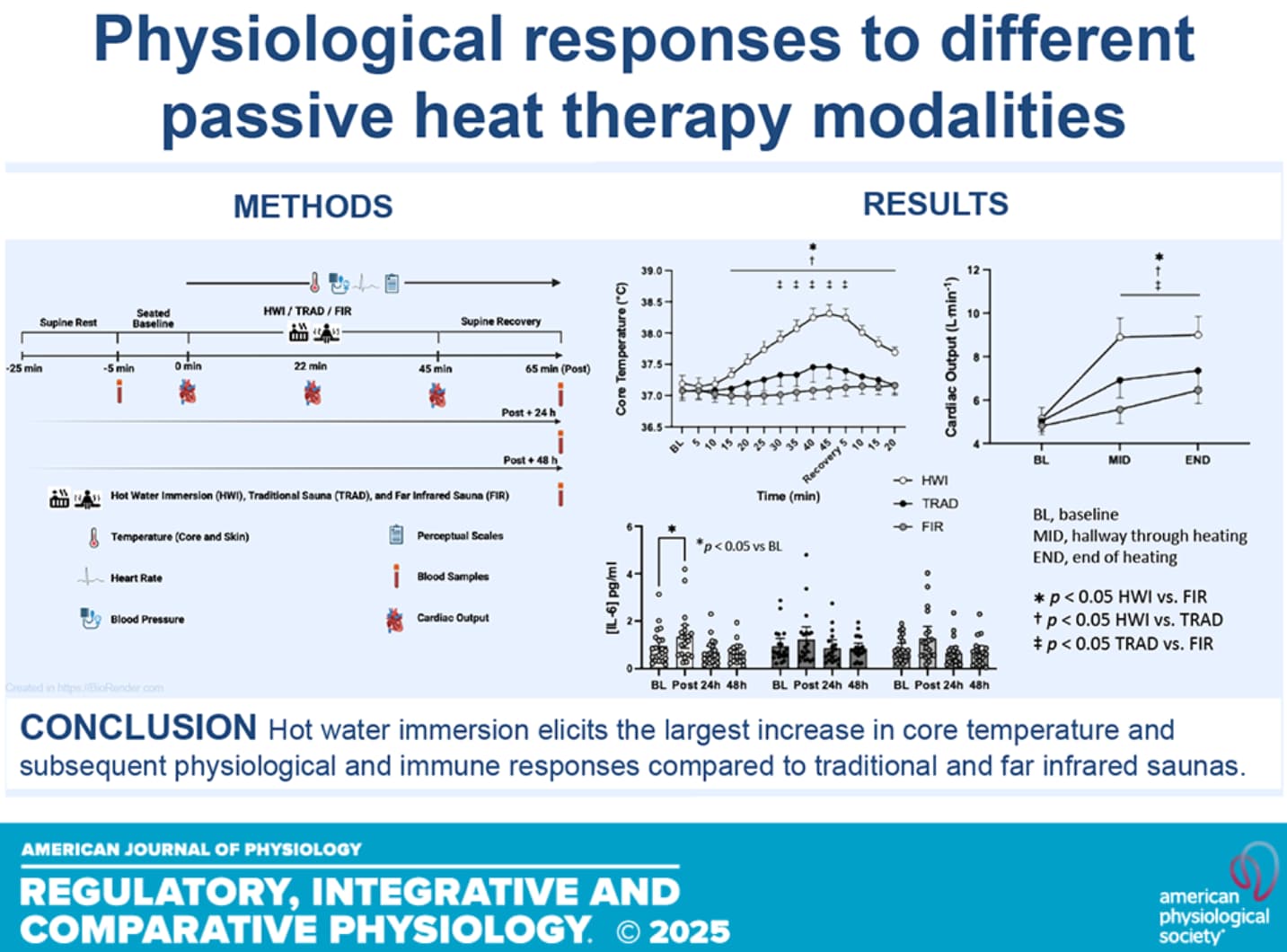
During hot water immersion, participants were immersed to the level of the sternum in ∼40.5°C water for 45 min. Participants’ left arm was supported above the water for blood pressure measurements.
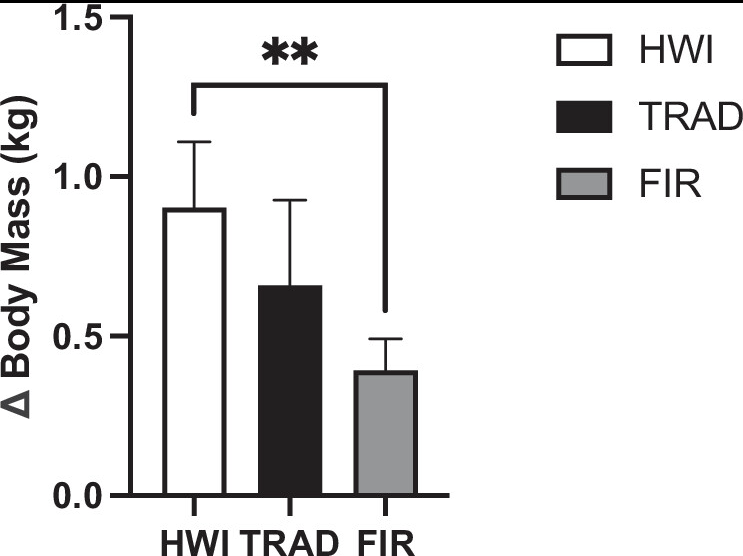
Figure 3. Total sweat loss in hot water immersion (HWI), traditional sauna (TRAD), and far infrared sauna (FIR). **P < 0.01 HWI vs. FIR and TRAD.
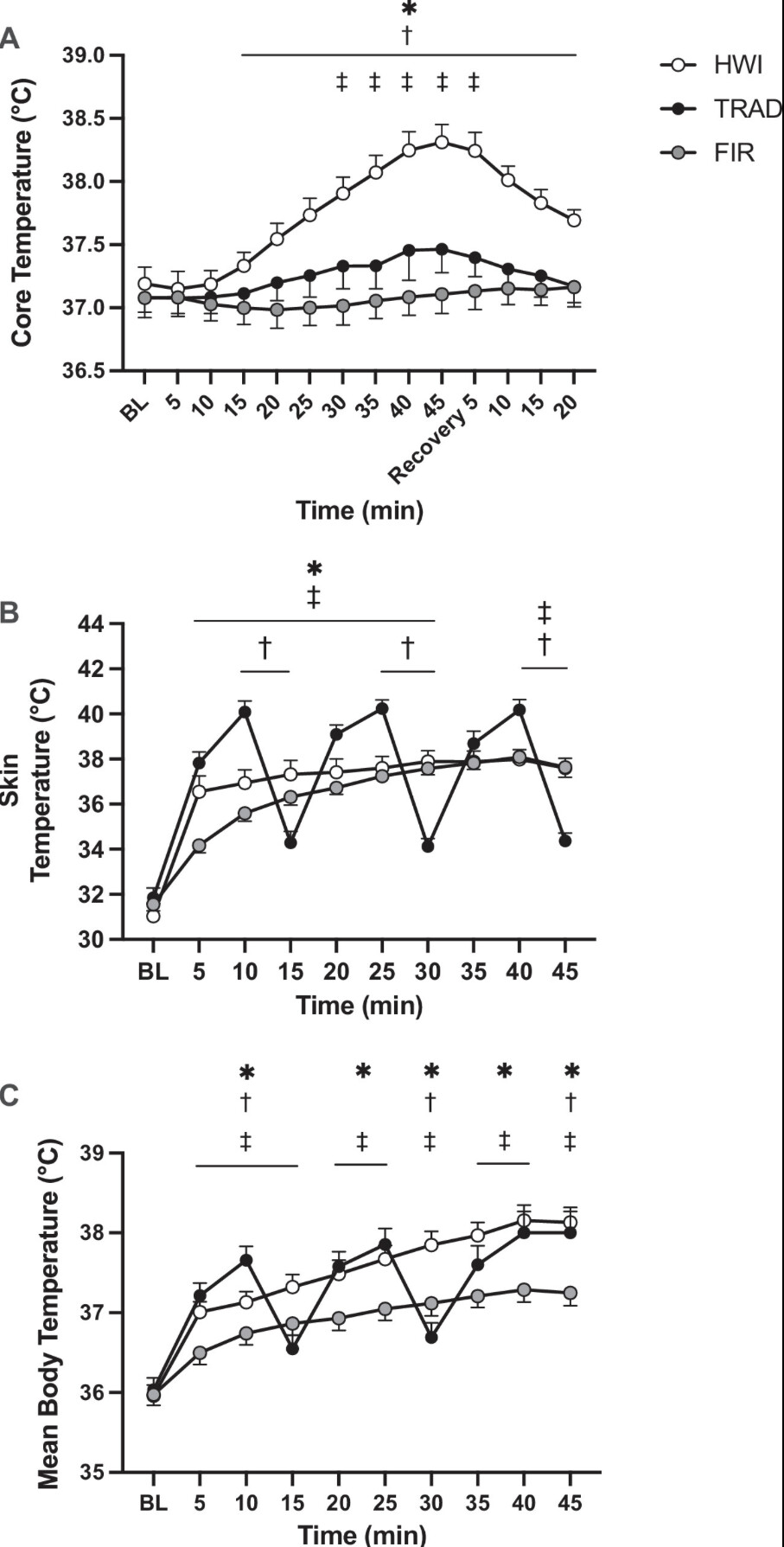
Figure 2. Core temperature (A ) at baseline (BL), throughout heating, and recovery in hot water immersion (HWI), traditional sauna (TRAD), and far infrared sauna (FIR). Skin temperature (B ) and mean body temperature (C ) at baseline and throughout heating. *P < 0.05 HWI vs. FIR, †P < 0.05 HWI vs. TRAD, ‡P < 0.05 TRAD vs. FIR.
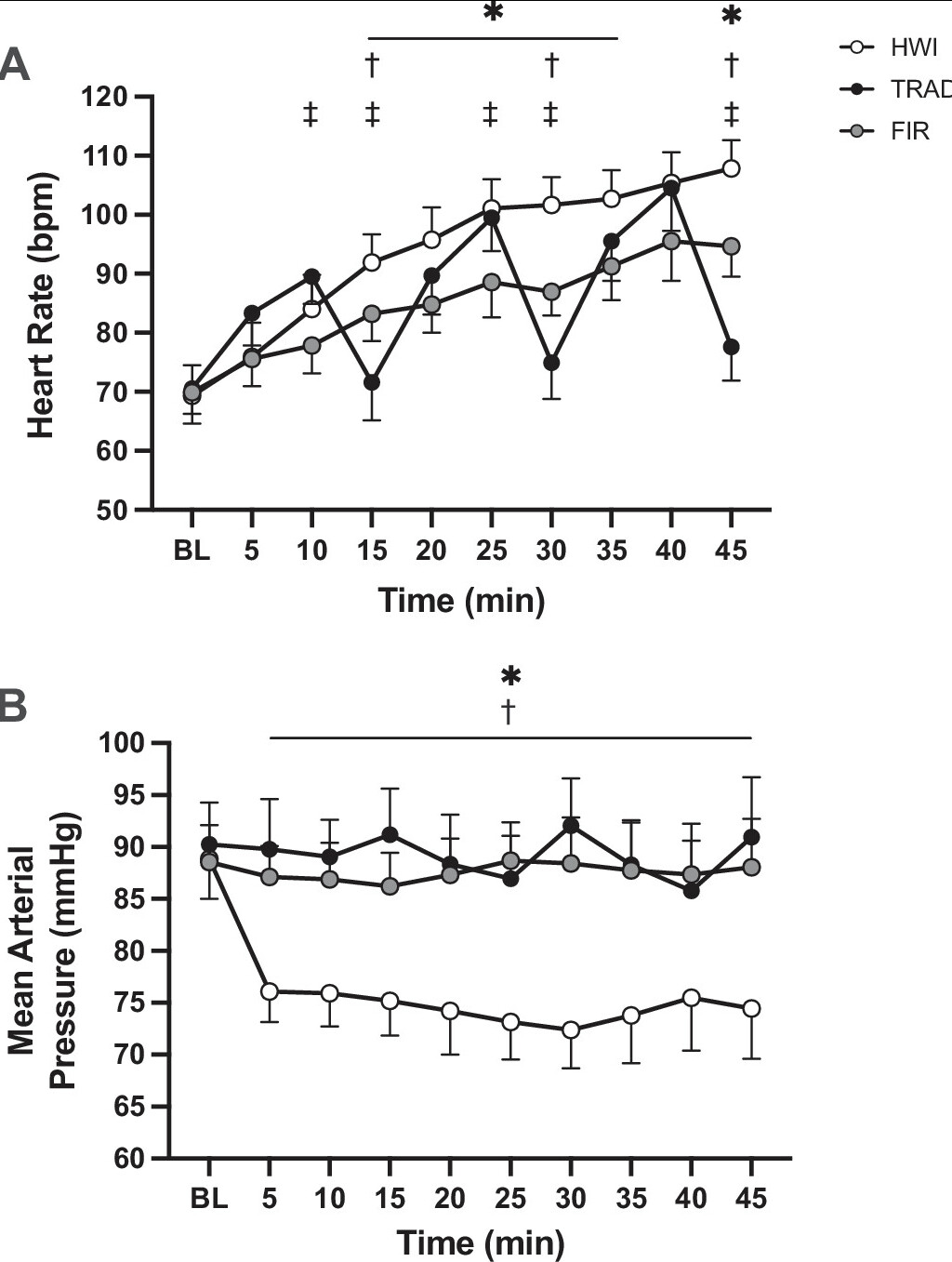
Figure 4. Heart rate (A ) and mean arterial pressure (B ) at baseline and throughout heating in hot water immersion (HWI), traditional sauna (TRAD), and far infrared sauna (FIR). *P < 0.05 HWI vs. FIR, †P < 0.05 HWI vs. TRAD, ‡P < 0.05 TRAD vs. FIR.
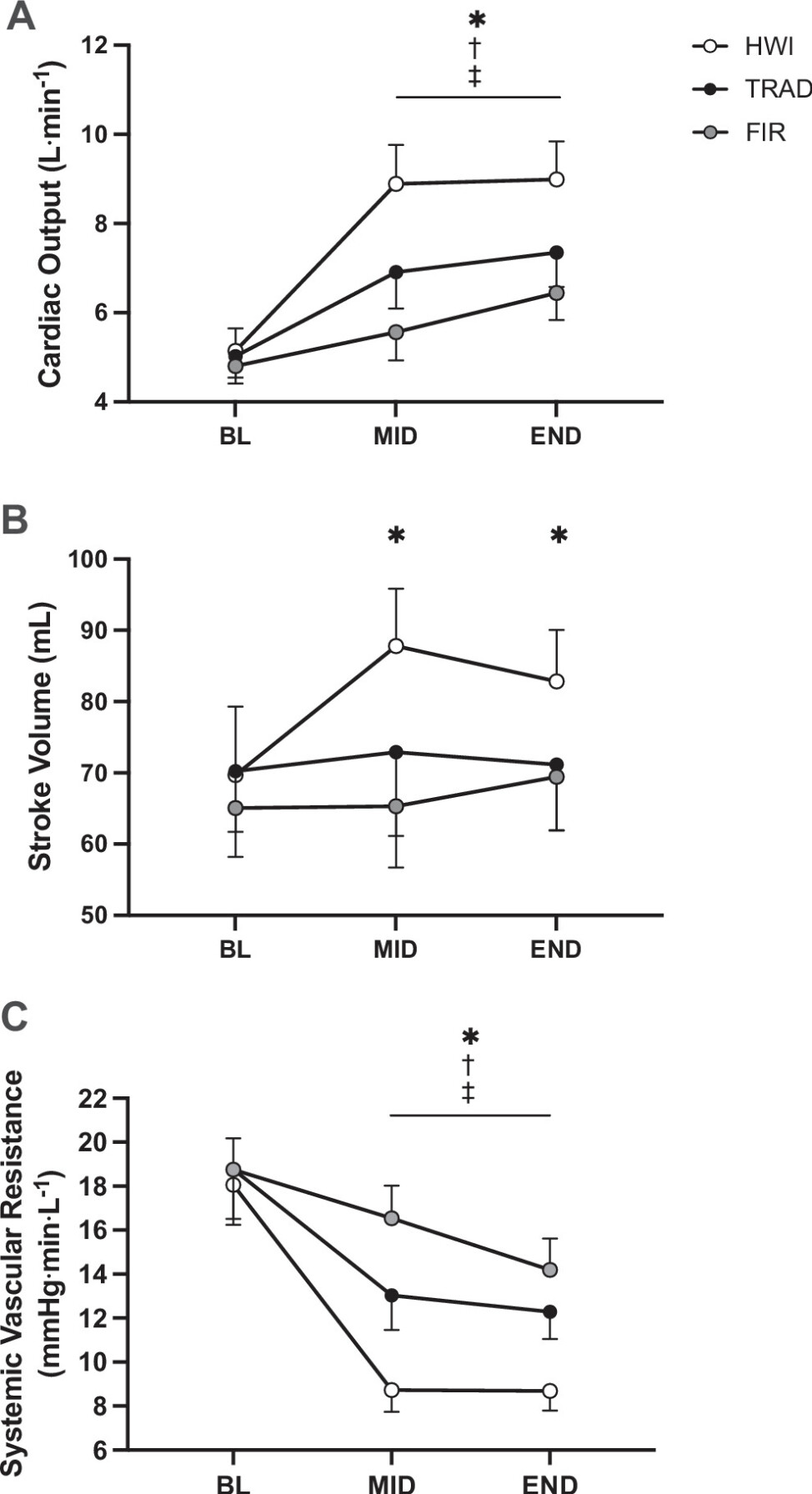
Figure 6. Cardiac output (A ), systemic vascular resistance (B ), and stroke volume (C ) at baseline (BL), halfway through heating (MID), and at the end of hearting (END) in hot water immersion (HWI), traditional sauna (TRAD), and far infrared sauna (FIR). *P < 0.05 HWI vs. FIR, †P < 0.05 HWI vs. TRAD, ‡P < 0.05 TRAD vs. FIR.