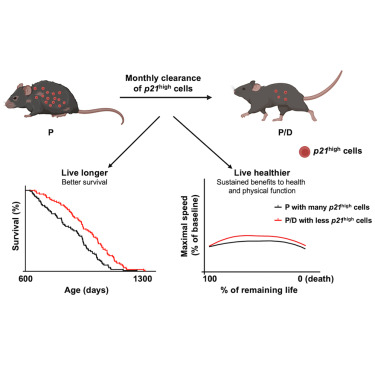
Our study implies that p21high cells might be a potential therapeutic target for healthspan extension. Monthly clearance of p21high cells not only extends lifespan by 9% but also improves physical function at all stages of post-treatment life, including the late life close to death. Postmortem pathological examinations reveal that clearance of p21high cells does not change disease burden from a qualitative or quantitative perspective and severity despite longer lifespan. This suggests that the lifespan and healthspan extension might be due to slowing of the aging process rather than selectively alleviating individual-specific disease processes. Slowing of the aging process is further supported by the maximum lifespan extension following clearance of p21high cells. It is important to note that clearance of p21high cells using our p21-Cre mouse model does not interrupt p21 gene function since p21 is only leveraged as a marker to target a small number of cells in our model. Indeed, inactivation of p21 leads to reduced lifespan with increased tumor incidence, which is opposite to what we observed in P/D mice.
In our study, we chose 20 months as the treatment starting point instead of middle age because (1) p21high cells do not accumulate in tissues at the middle age, and treating middle aged P and P/D mice with tamoxifen (to clear p21high cells if any) does not have any observable benefit on physical function the median lifespans for P and P/D mice are 30 and 32.5 months, respectively—therefore, initiating intervention at 20 months ensures a remaining lifespan of over 10 months on average, providing a sufficient timeframe for detecting any potential long-term effects; and (3) starting intervention later in life might hold better translational potential than starting early. For example, our study indicates that even clearing p21high cells later in life yields profound benefits, requiring shorter treatment durations and fewer doses compared with starting at middle age.
One hallmark of aging is persistent and low-grade “sterile” inflammation, which is highly associated with frailty and other age-related conditions. The underlying mechanisms of age-related chronic inflammation remain elusive. Our findings suggest that p21high cells might be a major contributor. Various p21high cell types share a conserved proinflammatory signature, which can spread and amplify inflammation within the microenvironment. Elimination of p21high cells reduces inflammation in liver, fat, muscle, and heart, as well as circulating cytokines, without affecting circulating immune cell composition. Inflammation reduction might represent one major mechanism for healthspan extension in P/D mice, along with others to be examined.
Both p21 and p16 are widely used as senescence markers in most studies, and p21high cells indeed exhibit a number of key features of senescent cells, including enlarged size, increased SA-β-gal activity, reduced proliferative capacity, lower lamin B1 expression, and various altered pathways associated with senescence. However, emerging evidence suggests that senescent cells are highly heterogeneous, and p21high and p16high cells are distinct cell populations in various tissues in vivo. Importantly, the role of p21high cells in aging seems to be different from p16high cells or cells targeted by senolytic drugs (dasatinib + quercetin [D + Q]). Although both D + Q and clearance of p16high cells using INK-ATTAC mice extended median lifespan in C57BL/6 mice, neither of these interventions extended maximum lifespan, which was indeed extended by clearance of p21high cells. Moreover, using the same statistical analysis, we show that neither clearance of p16high cells nor D + Q extended healthspan, while clearance of p21high cells did. In fact, clearance of p16high cells demonstrates a weak and non-significant trend toward reducing, as opposed to extending healthspan in later life. These findings demonstrate that senescence-like cells targeted by different interventions might have distinct biological roles in vivo, and more research is desired to further understand the heterogeneity of senescent cells.
In summary, our study provides proof-of-concept evidence that intermittent clearance of p21high cells can extend lifespan and healthspan in aged mice. Although p21high cells are heterogeneous in cell type, several transcriptomic signatures seem to be relatively conserved among various cell types, which can be leveraged to develop new pharmacological interventions to specifically target these cells. Depending on future drug developments and clinical trials, targeting p21high cells might hold great promise for slowing down the aging process and promoting healthy aging.