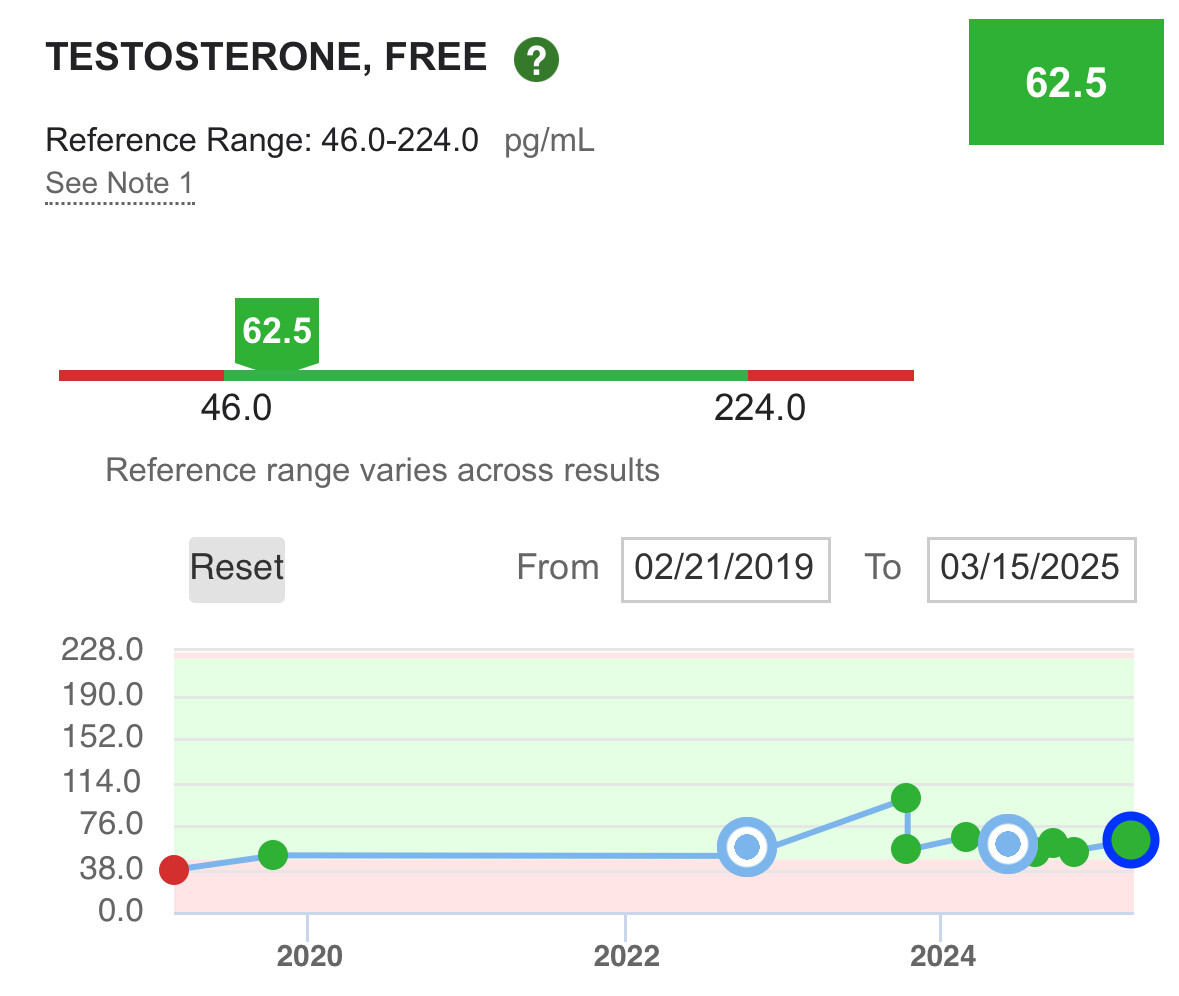
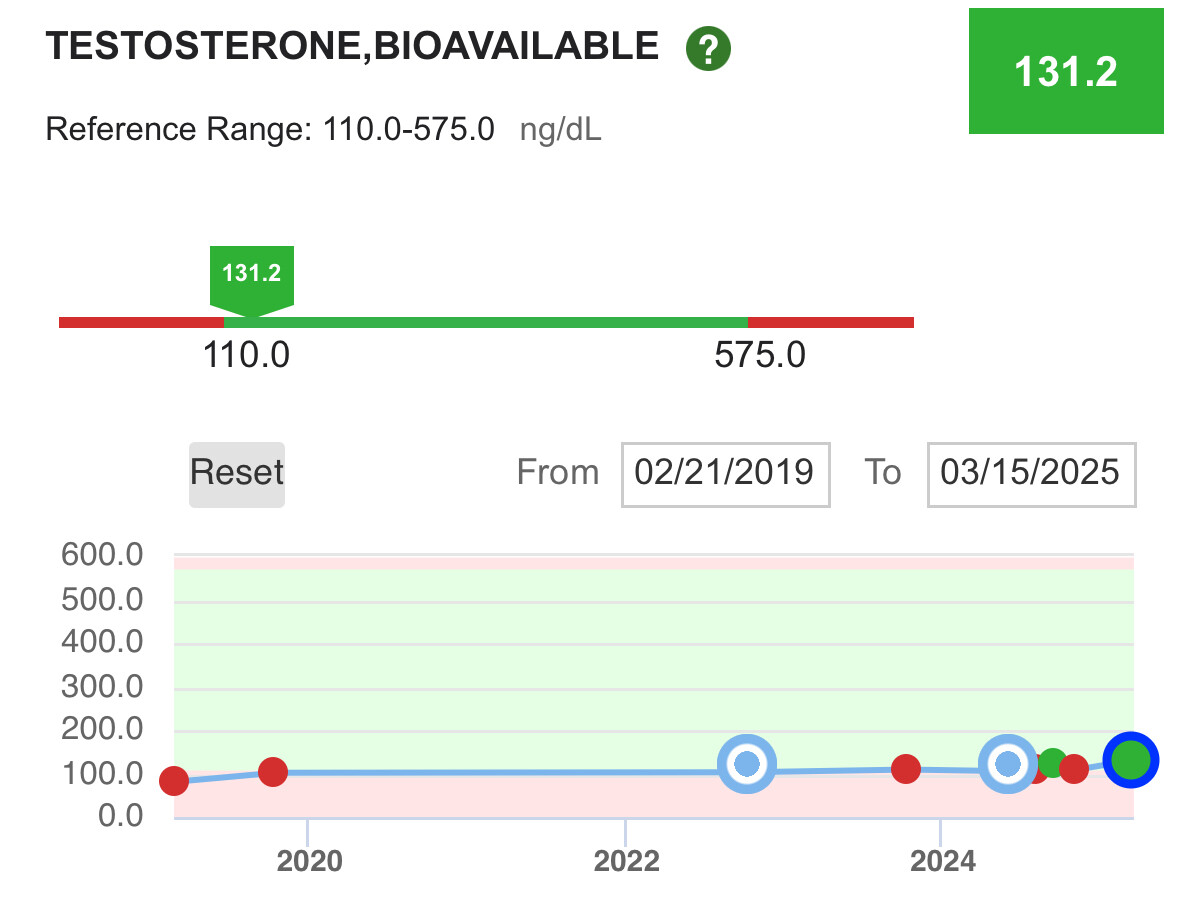
One of the reasons I use secretagogues (peptides that tell the hormone system to make hGH) instead of somatotropin (the synthetic version of hGH) is to more closely mimic how my body worked when I was younger. That can be done with somatotropin with proper timing but it bypasses the function of the pituitary gland, i.e. a replacement therapy. My personal goal is to have all my organs doing their job, not replace their function.
For people with loss of pituitary function, replacement therapy is the only way to go.
GH is naturally released in a pulsatile manner, it is not naturally present at higher levels throughout the day. This is what secretagogue peptides do, when used to support the natural circadian hormone cycle.
This is also why I choose to use CJC 1295 noDAC as opposed to the DAC versions. The DAC version has a much longer half-life and keeps the GH levels high much longer. The noDAC version has a 2 hr half-life and we take it (in combination with Ipamorelin) just before bedtime. Approximately 2 hours into sleep is when our body produces a GH peak, As we age this peak declines, as does IGF-1.
The circulating half-life of hGH is relatively short (20-30 minutes), while its biological half-life is much longer (9-17 hours).
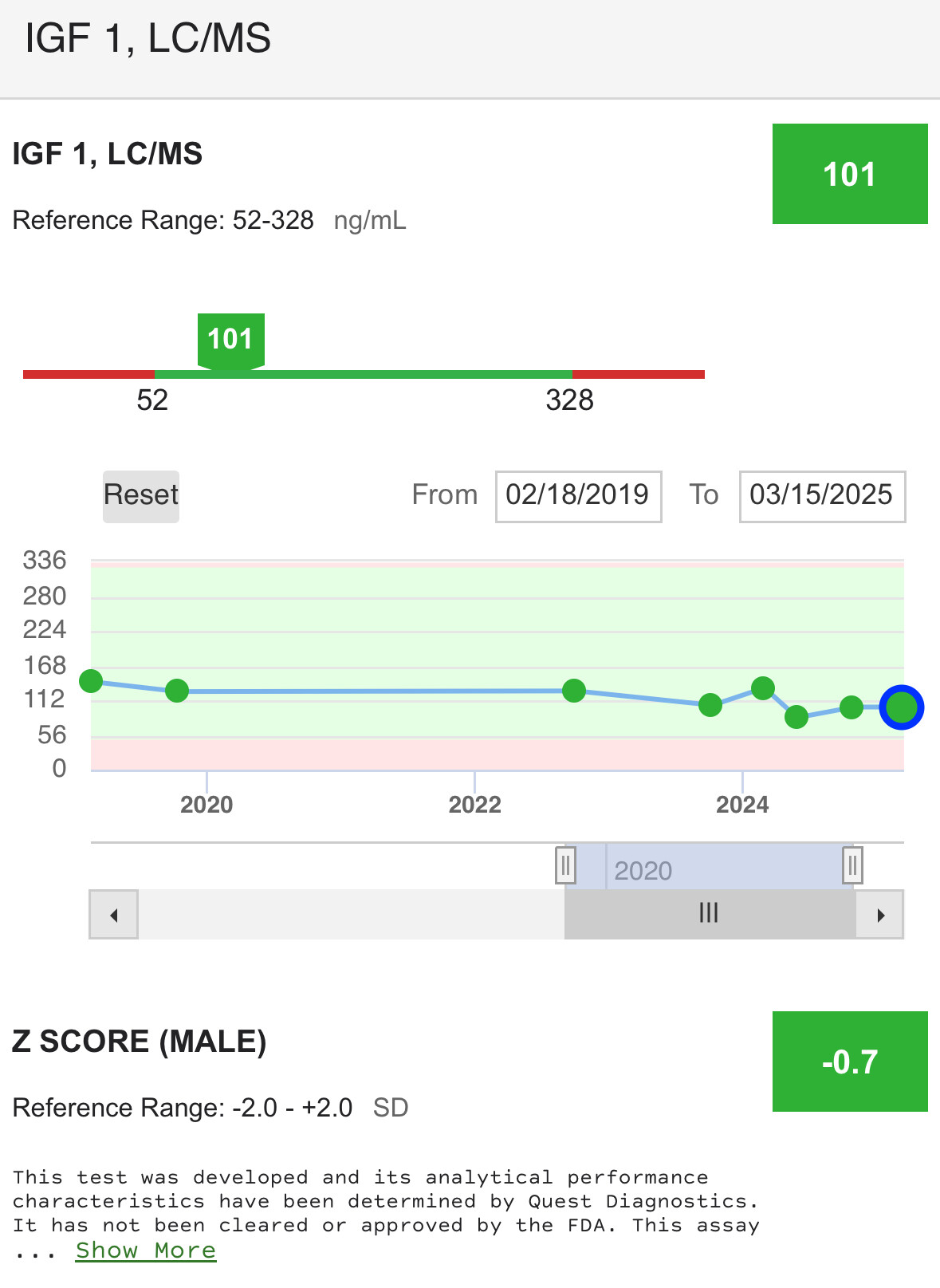
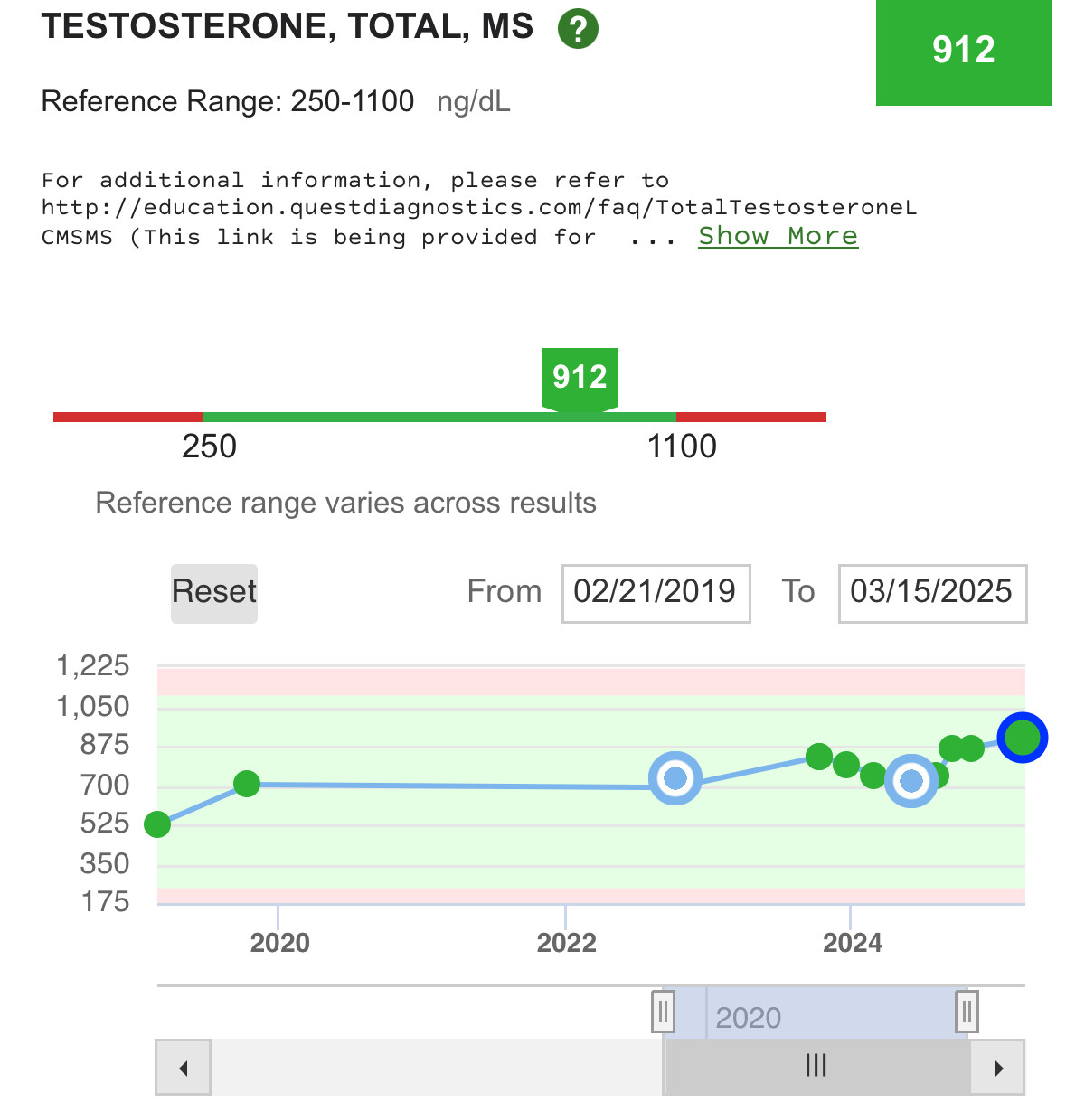
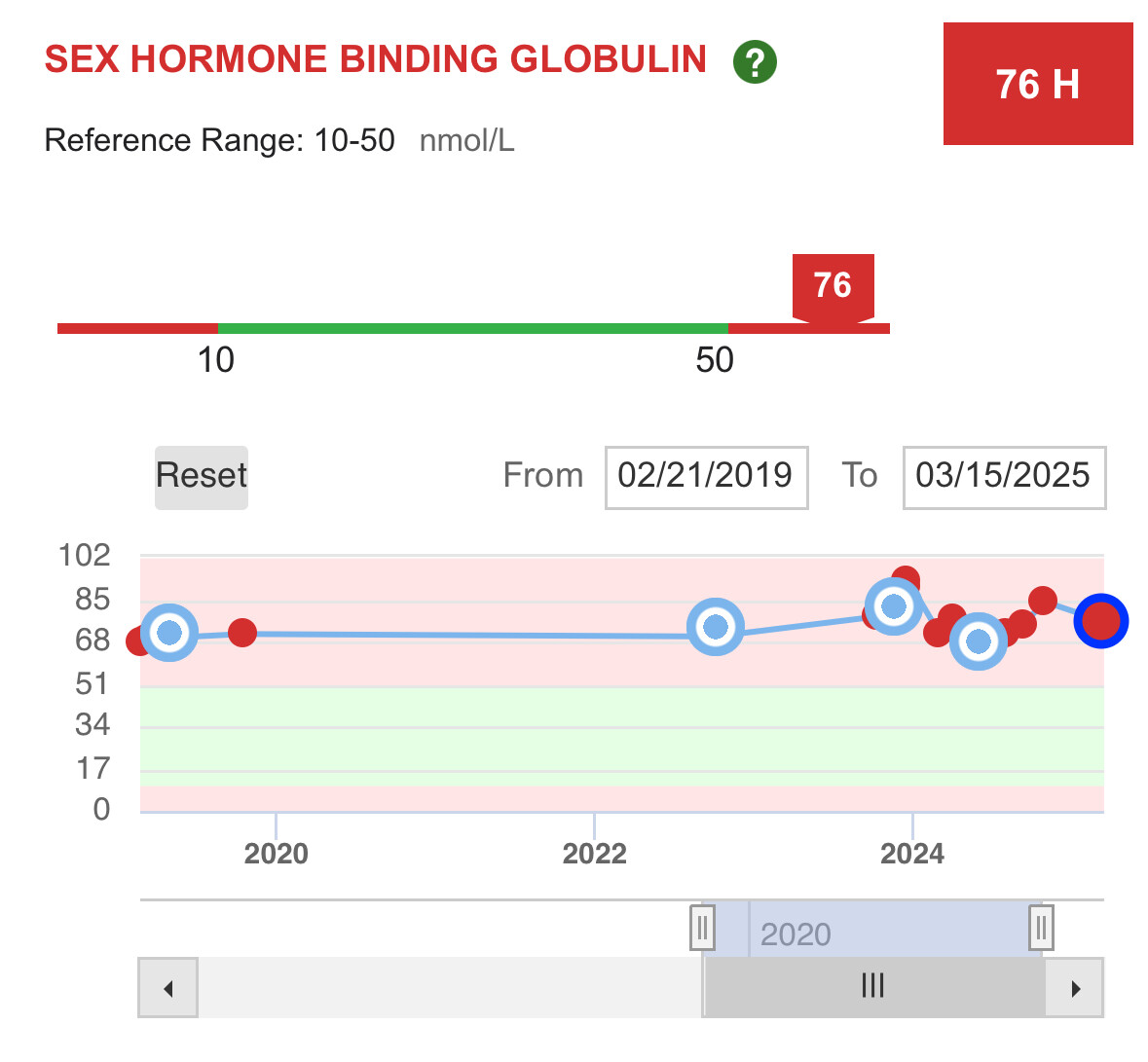
When GH goes high, so does IGF-1 to normalize the GH levels to mitigate insulin resistance that GH causes. This is why there is an insulin management compound required for the TRIIM protocol where they use Metformin for this purpose.
When GH goes low, so does IGF-1. They are a synergistic feedback pair.
As IGF-1 levels seem to be used as a proxy for GH levels in blood tests and that low IGF-1 levels are associated with longevity, thereby indicating that low GH levels by proxy are desired, I wonder if the insulin interplay is being missed as part of the “longevity” equation in this triple-play of interactions. We know that poorly controlled insulin levels (not even with diagnosed T2D, even before that presents) are an issue for longevity and that the earlier in life these levels are normalized the greater the benefit for health span.
So who is the bad guy in this equation? hGH? IGF-1? Insulin?
In conclusion, GH stimulates IGF-1 levels and insulin concentrations, the latter mainly by inducing insulin resistance. IGF-1 decreases GH levels by growth hormone releasing hormone (GHRH)-dependent feedback mechanisms. Insulin increases liver GHR expression, making the liver more GH-sensitive, leading to an increase in IGF-1 and a decrease in GH. On the other hand, low portal insulin levels (e.g., during prolonged fasting) reduce hepatic GHR expression, thereby reducing IGF-1 levels and increasing GH concentrations by the lack of IGF-1 feedback on pituitary GH secretion
https://www.e-enm.org/journal/view.php?doi=10.3803/EnM.2024.101