This is really an interesting case of “a tale of two studies”, where one somewhat contradicts the other one to some degree.
First we have this:
GLP-1RA and SGLT2i Medications for Type 2 Diabetes and Alzheimer Disease and Related Dementias
https://jamanetwork.com/journals/jamaneurology/article-abstract/2831976
“Results This study included 33 858 patients in the GLP-1RA vs other GLD cohort (mean age, 65 years; 53.1% female), 34 185 patients in the SGLT2i vs other GLD cohort (mean age, 65.8 years; 49.3% female), and 24 117 patients in the GLP-1RA vs SGLT2i cohort (mean age, 63.8 years; 51.7% female). In IPTW-weighted cohorts, the incidence rate of ADRD was lower in GLP-1RA initiators compared with other GLD initiators (rate difference [RD], −2.26 per 1000 person-years [95% CI, −2.88 to −1.64]), yielding an HR of 0.67 (95% CI, 0.47-0.96). SGLT2i initiators had a lower incidence than other GLD initiators (RD, −3.05 per 1000 person-years [95% CI, −3.68 to −2.42]), yielding an HR of 0.57 (95% CI, 0.43-0.75). There was no difference between GLP-1RAs and SGLT2is, with an RD of −0.09 per 1000 person-years (95% CI, −0.80 to 0.63) and an HR of 0.97 (95% CI, 0.72-1.32).”
So here we see robust HR lowering for both GLP-1RAs and SGLT2is, and the SGLT2i certainly didn’t come off worse and perhaps even marginally better.
Which brings us to the second paper:
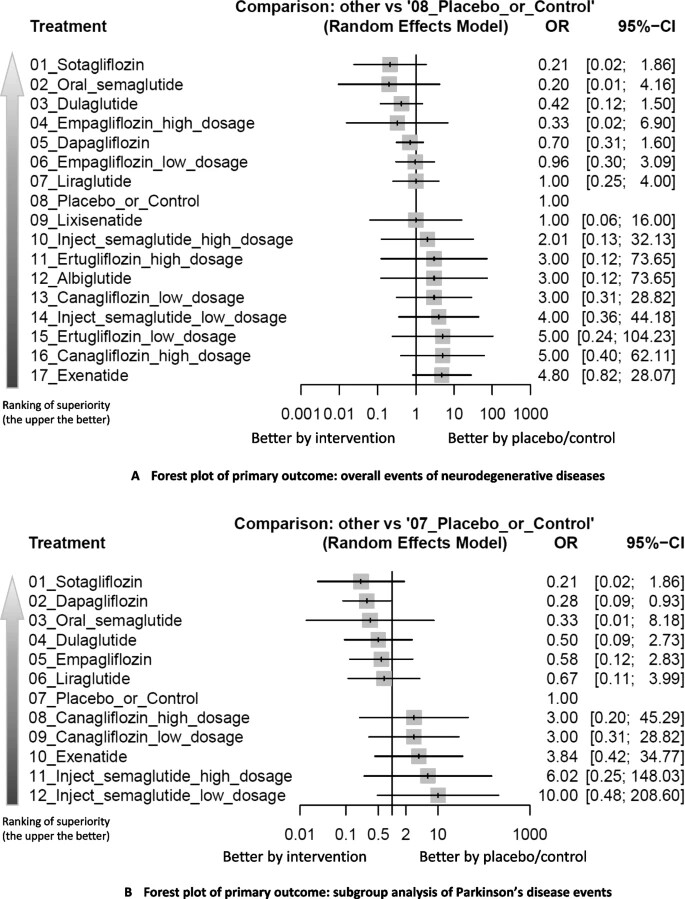
Cardioprotective Glucose-Lowering Agents and Dementia RiskA Systematic Review and Meta-Analysis
https://jamanetwork.com/journals/jamaneurology/fullarticle/2831975
“Among drug classes, glucagon-like peptide-1 receptor agonists (GLP-1RAs) were associated with a statistically significant reduction in dementia, but not sodium-glucose cotransporter-2 inhibitors (SGLT2is).”
And there you have it. One can look at the differences between these to try to account for why one found an effect with SGLT2is while the other did not. Perhaps duration was a key factor.