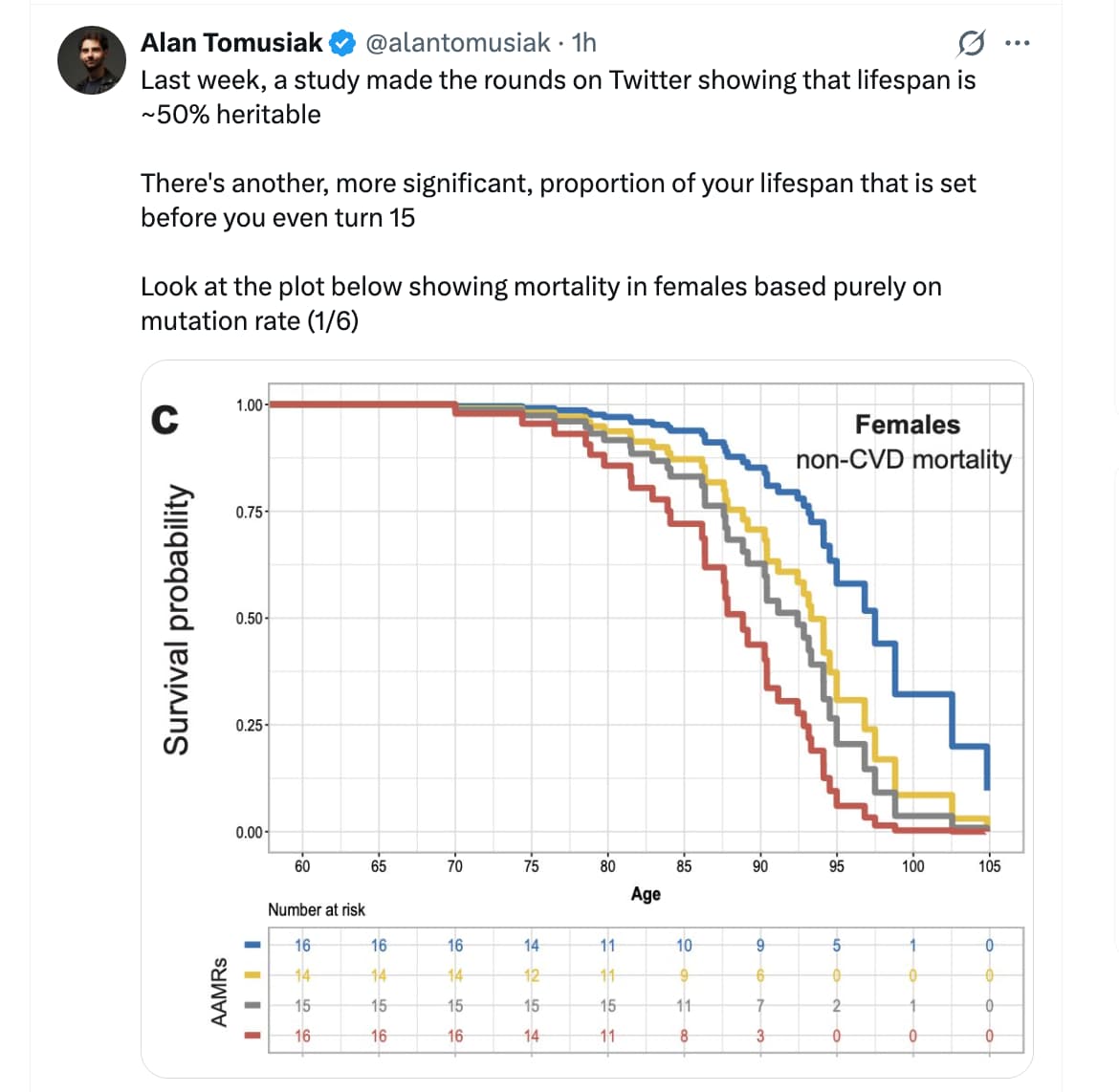
Scientists may have discovered a biological crystal ball hidden within our reproductive cells. An important study conducted at the University of Utah (USA) and published in the journal Scientific Reports reveals that the rate at which young adults accumulate mutations in their germline—the DNA passed to offspring—acts as a powerful predictor of both total lifespan and, for women, reproductive longevity.
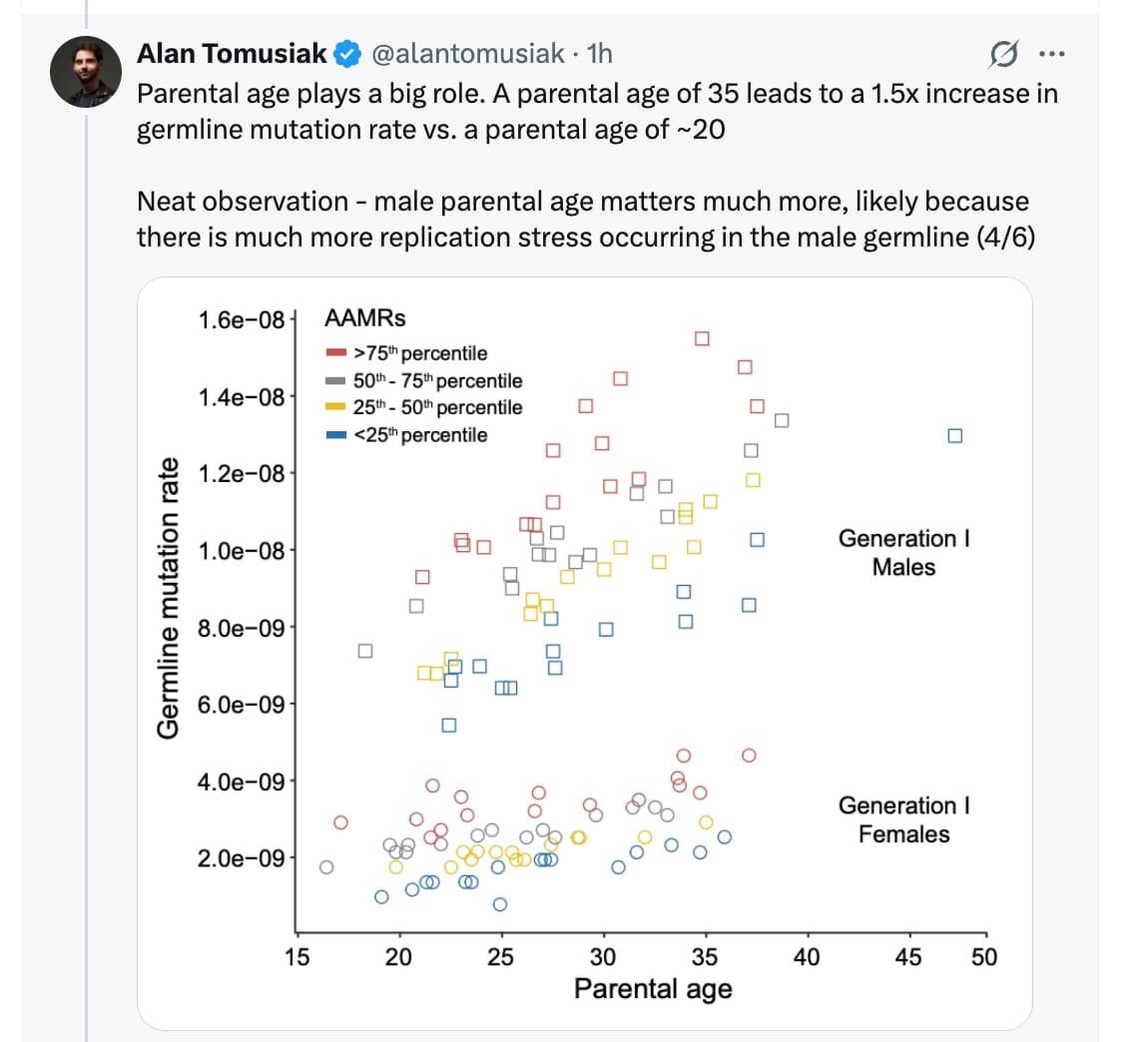
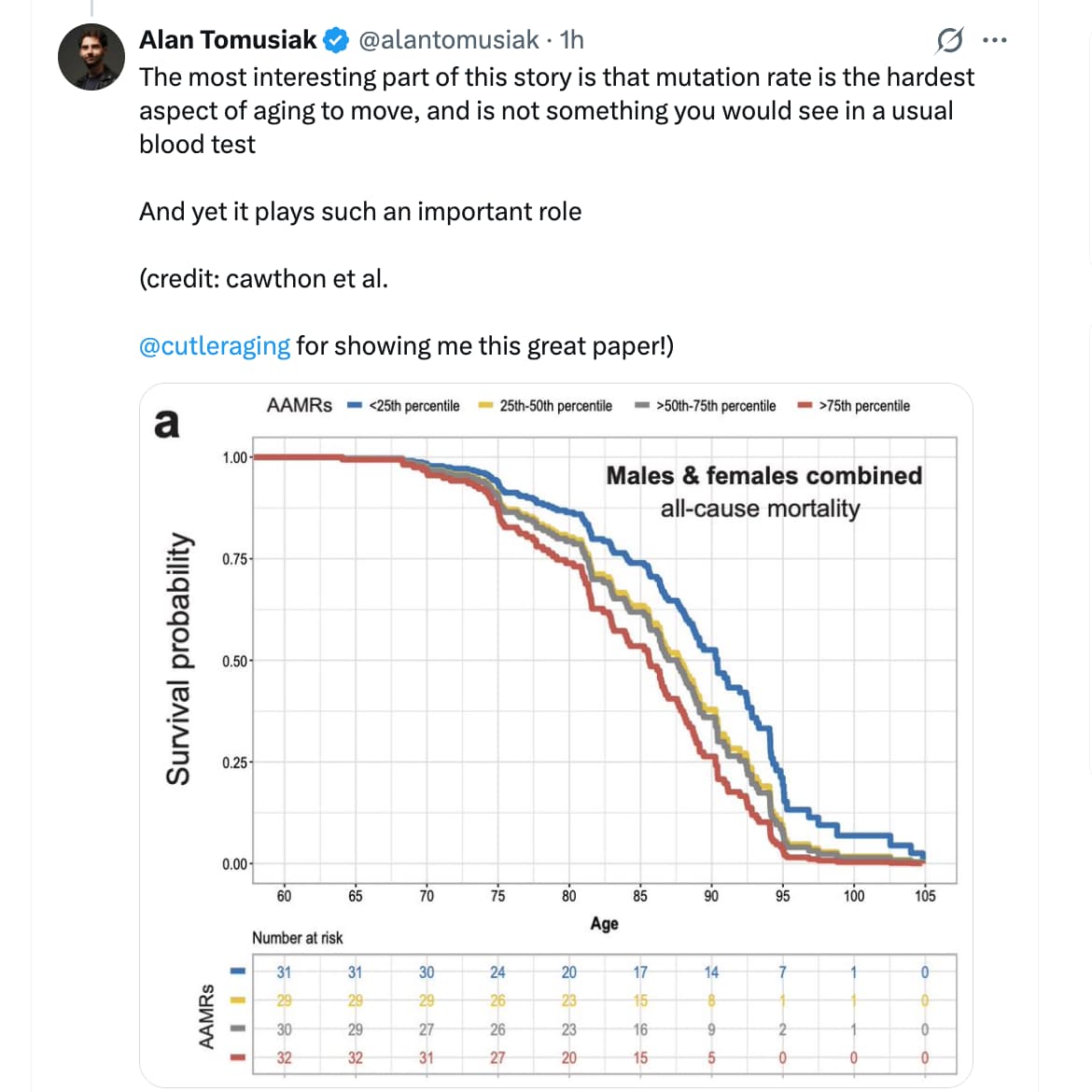
The research team analyzed 121 individuals from the Utah CEPH families, using high-depth whole-genome sequencing of three-generation pedigrees to calculate “Age-Adjusted Mutation Rates” (AAMRs). The findings were stark: individuals in the highest quartile of mutation accumulation experienced more than double the mortality risk compared to those in the lowest quartile. This translates to a median survival difference of nearly five years—a gap comparable to the impact of chronic smoking or diabetes.
The “Big Idea” here is that the germline isn’t just a passive vessel for heredity; it is a high-fidelity mirror of an individual’s systemic DNA repair efficiency. Because germ cells are sequestered early in development and must maintain extreme integrity to ensure viable offspring, they serve as a “clean” environment to measure the baseline rate of genetic decay. The study suggests that if your body is “sloppy” at repairing DNA in your sperm or eggs during your 20s, it is likely equally sloppy at maintaining your heart, brain, and lungs, leading to accelerated systemic aging.
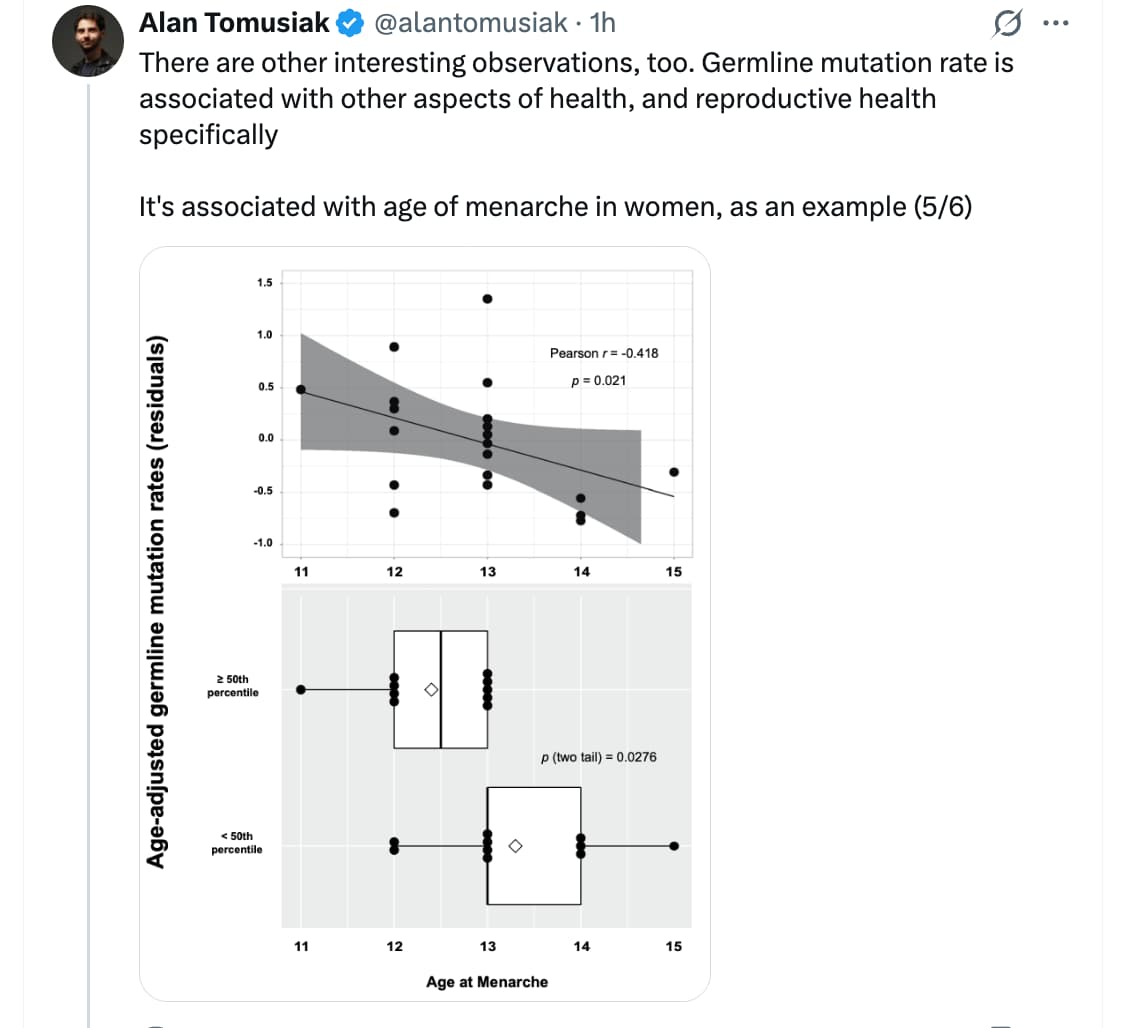
For women, the clock is even more specific. A higher mutation rate strongly correlated with an earlier “Age at Last Birth” (ALB), suggesting that the same mechanisms protecting the genome from mutations also preserve the ovarian reserve. Interestingly, the study pinpointed puberty as the critical “on-switch” for these adult mutation rates, with late-blooming girls showing lower lifetime mutation accumulation.
Source:
- Open Access Paper: Germline mutation rates in young adults predict longevity and reproductive lifespan
- Journal/Date: Nature Scientific Reports, 2020
- Impact Evaluation: The impact score of this journal is ~3.9, evaluated against a typical high-end range of 0–60+ for top general science, therefore this is a Medium impact journal. While not “Elite” like Nature or Science, it is a high-volume, rigorously peer-reviewed member of the Nature Portfolio that carries significant weight in the genomics community.
Part 2: The Biohacker Analysis (Style: Technical, Academic, Direct)
Study Design Specifications
- Type: In vivo human longitudinal cohort analysis (Pedigree-based).
- Subjects: 121 “Generation I” individuals (61 females, 60 males) from the Utah CEPH families.
- Methodology: Trio-based whole-genome sequencing (WGS) of parents and offspring to identify de novo mutations (DNMs).
- Lifespan Analysis: This study did not utilize mouse models; however, it validates the “Somatic Mutation Theory of Aging” in humans, echoing findings that species with lower mutation rates (like humans vs. mice) have higher maximum lifespans Population size and germline mutation rate (2023).
Lifespan Data (Human)
- Hazard Ratio: 2.07 (95% CI, 1.21–3.56) for all-cause mortality in the top vs. bottom AAMR quartile.
- Absolute Time: Median survival difference of 4.7 years between quartiles.
Mechanistic Deep Dive The study identifies the DNA Damage Response (DDR) as the primary longevity driver.
- Target Pathways: The findings implicate Nucleotide Excision Repair (NER) and **Base Excision Repair (BER)**efficiency.
- Organ-Specific Priority: Unlike somatic mutation studies that focus on cancer-prone tissues (e.g., skin, colon), this suggests a germline-soma symmetry. The “mutator phenotype” expressed in the germline is a proxy for systemic failure in maintaining genomic stability across all post-mitotic tissues.
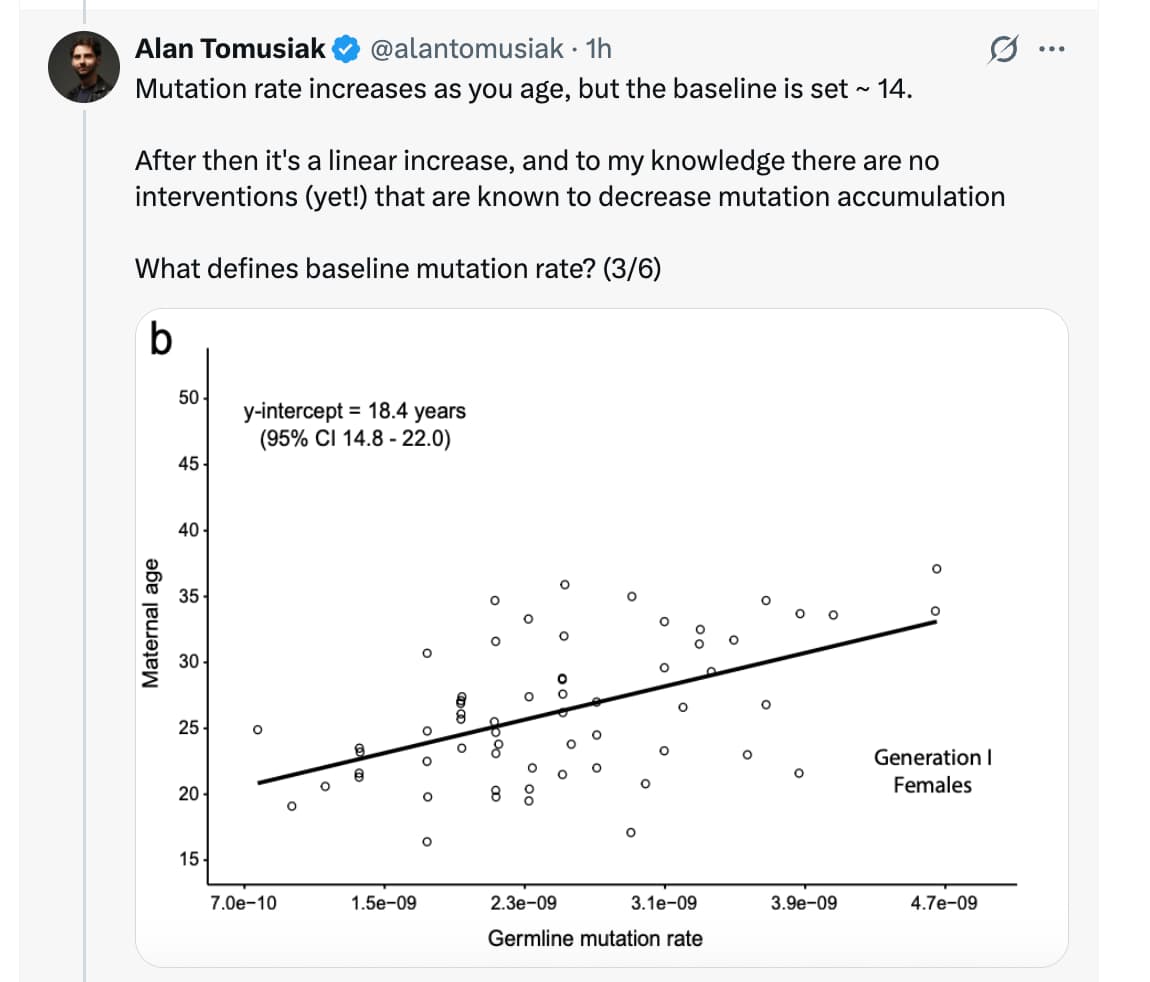
- Puberty/Hormonal Trigger: The study posits that the establishment of adult mutation rates occurs between ages 14 (males) and 18 (females). This suggests that IGF-1/Growth Hormone surges during puberty may downregulate high-fidelity repair mechanisms in favor of rapid growth.
Novelty This is the first study to prove that germline mutation rates in young, healthy adults—measured decades before death—can predict actual human lifespan. It moves the “Mutation Theory of Aging” from a species-wide observation to an intra-species predictive tool.
Critical Limitations
- Sample Size: N=121 is relatively small for a mortality study, though the use of 3-generation pedigrees provides high-accuracy mutation data.
- Translational Gap: The study measures an intrinsic rate, not an intervention. We do not yet know if increasing DNA repair efficiency in adulthood can “reset” this clock once established at puberty.
- Cause of Death: While all-cause mortality was significant, the strongest female correlation was with non-cardiovascular death, suggesting the mechanism may be more relevant to cancer or general frailty than to vascular health.
Part 3: Hierarchy of Evidence & Claim Verification
| Claim | Evidence Level | Verification Status | Translational Label |
|---|---|---|---|
| High germline mutation rates predict shorter lifespan. | Level C | Cawthon et al. (2020) | Human (Validated) |
| Mutation rates are established during puberty. | Level C | Cawthon et al. (2020) | Human (Validated) |
| Lower IGF-1/IIS activity improves DNA repair. | Level D | IIS Pathway and Longevity (2012) | Translational Gap(Animal data) |
| Higher mutation rates shorten female reproductive lifespan. | Level C | Cawthon et al. (2020) | Human (Validated) |
| KIF2C agonists can enhance DNA repair. | Level D | KIF2C and DNA repair (2025) | Translational Gap (In vitro) |
Safety Check: There are currently no FDA-approved “DNA repair enhancers.” Most compounds targeting these pathways are in oncology as inhibitors (e.g., PARP inhibitors).