Researchers at the University of Arkansas for Medical Sciences have published a study in 2024 suggesting that Ezetimibe, a common FDA-approved cholesterol-lowering drug, may drastically reduce the risk of Alzheimer’s Disease (AD) and related dementias. The central finding is not related to the drug’s primary function of lipid management, but rather a newly identified off-target effect: the physical disruption of a specific protein interaction that stabilizes toxic aggregates in the brain.
The study identifies a pathological “handshake” between the signaling protein 14-3-3 gamma and the mitochondrial enzyme Hexokinase-1 (HK1). This interaction appears unique to AD and heart-disease-affected brains. The researchers propose that this binding event acts as a “lynchpin,” stabilizing toxic protein aggregates and preventing the cell’s autophagy system from clearing them. Through computational screening and biological validation, Ezetimibe was found to bind directly to the interface of these two proteins, effectively blocking their interaction and destabilizing the aggregates.
In a retrospective analysis of insurance claims data covering nearly one million patients, those prescribed Ezetimibe showed a relative risk of 0.14 for developing Alzheimer’s compared to matched controls—a roughly seven-fold reduction in incidence. This effect size is significantly larger than that observed with statins (approx. 32% reduction), suggesting the mechanism is distinct from simple cholesterol lowering. While the magnitude of this reduction in the clinical data is anomalously high and likely influenced by confounding factors, the mechanistic validation in cell and nematode models offers a compelling biological rationale for the observation.
Ezitimibe (Zetia) is an inexpensive, generic medication with a benign side effect profile that many people already take to help keep their cholesterol low.
Source
- Open Access Paper: Ezetimibe Lowers Risk of Alzheimer’s and Related Dementias over Sevenfold, Reducing Aggregation in Model Systems by Inhibiting 14-3-3G::Hexokinase Interaction
- Institution: University of Arkansas for Medical Sciences (UAMS), USA.
- Journal: Aging Biology., Published 2024 Jun 26
- Impact Evaluation This is an Emerging/Unranked impact journal. Readers should weigh the findings heavily on the methodological rigor presented rather than the prestige of the publication venue.
The Biohacker Analysis
Study Design Specifications
- Type: Multi-modal study including In silico docking, In vitro (human neuroblastoma SH-SY5Y cells), Ex vivo (human post-mortem tissue), In vivo (C. elegans), and Retrospective Clinical Cohort Analysis.
-
Subjects:
- Clinical Cohort: PharMetrics Plus database (2006–2020). Treatment Group: 4,361 patients on Ezetimibe. Control Group: ~945,000 matched controls (propensity score matched).
-
Animal Models: C. elegans strains:
- CL2355 (Neuronal A-beta 1-42 expression)
- VH255 (Pan-neuronal Tau expression)
- UA355 (A-beta 1-42 + APOE4)
- Human Tissue: Post-mortem hippocampus samples from AD patients, heart disease patients, and age-matched controls.
Lifespan Analysis
-
C. elegans Healthspan Data:
- Chemotaxis (Cognitive Function): In aged A-beta worms (CL2355), chemotaxis scores dropped to 37%. Ezetimibe treatment restored this to 72% (P < 0.0001).
- Paralysis (Proteotoxicity): In Tau-expressing worms (VH255), Ezetimibe delayed the onset of paralysis. At day 8, paralysis was reduced by 40%, and at day 13, it was reduced by 50% compared to controls.
- Neuronal Survival: In the APOE4 + A-beta model (UA355), Ezetimibe reduced neuronal loss by 10–20% (P < 0.03).
Mechanistic Deep Dive
The authors propose a “Aggregate-Lynchpin” hypothesis:
- The Target: The study isolates the 14-3-3 gamma :: HK1 protein complex as a critical stabilizer of insoluble aggregates.
- Mitochondrial Disruption: In AD pathology, Hexokinase-1 (HK1) detaches from the mitochondrial outer membrane (where it normally regulates VDAC/energy) and binds to 14-3-3 gamma in the cytoplasm.
- Action of Ezetimibe: Molecular docking simulations show Ezetimibe binds to the 14-3-3 gamma :: HK1 interface with a high binding energy (-9.7 kCal/mol). It physically blocks the two proteins from joining.
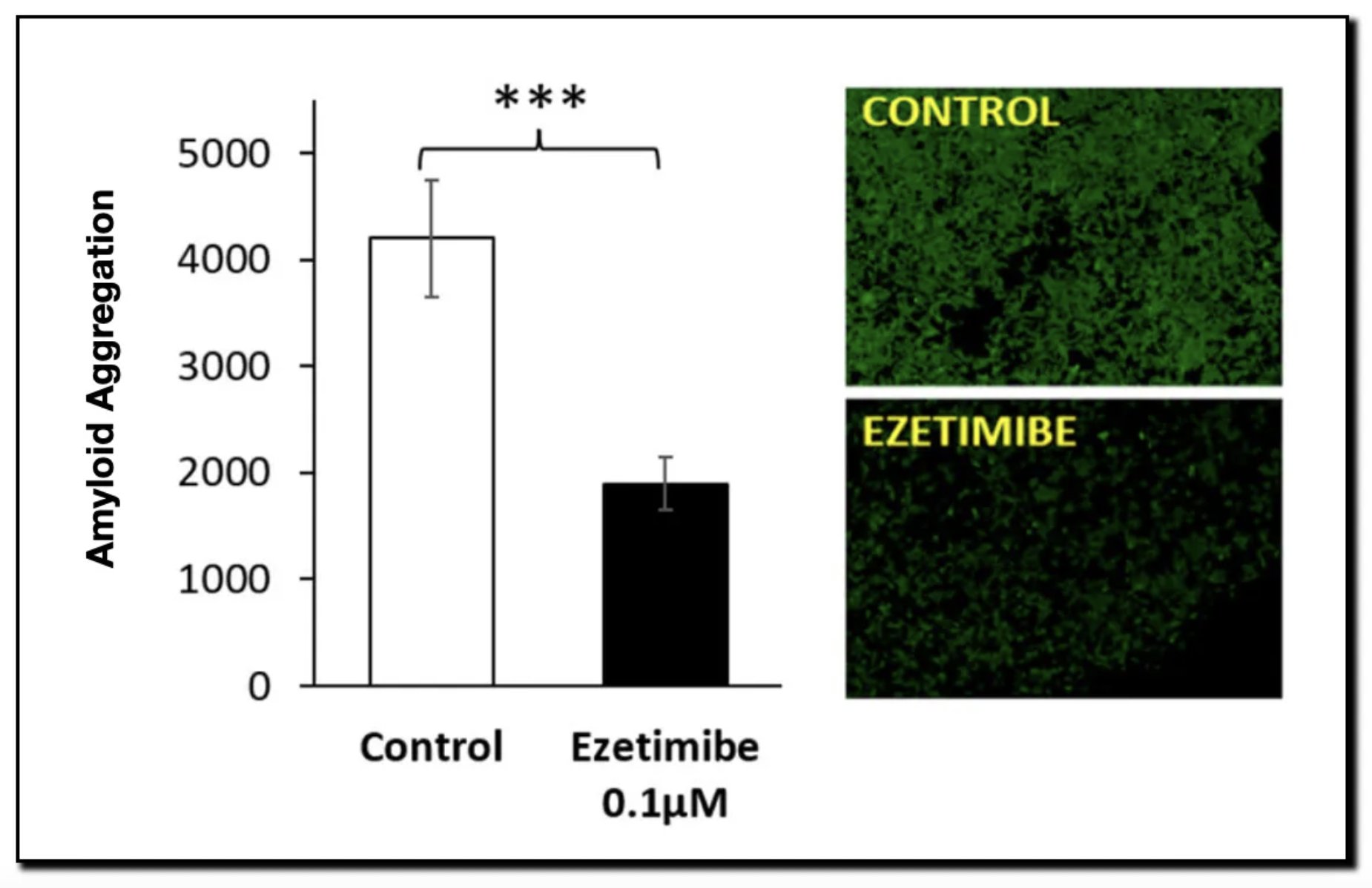
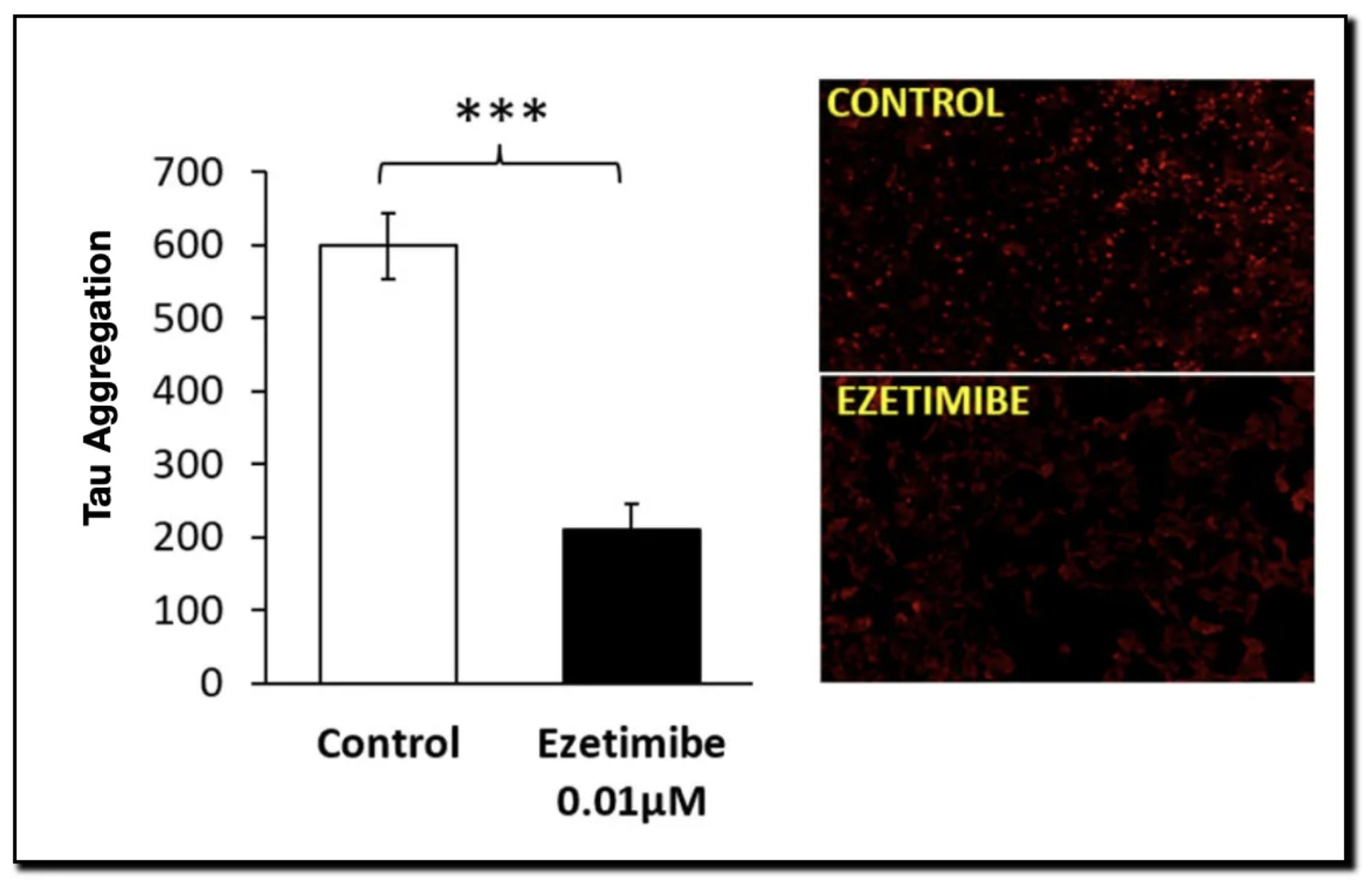
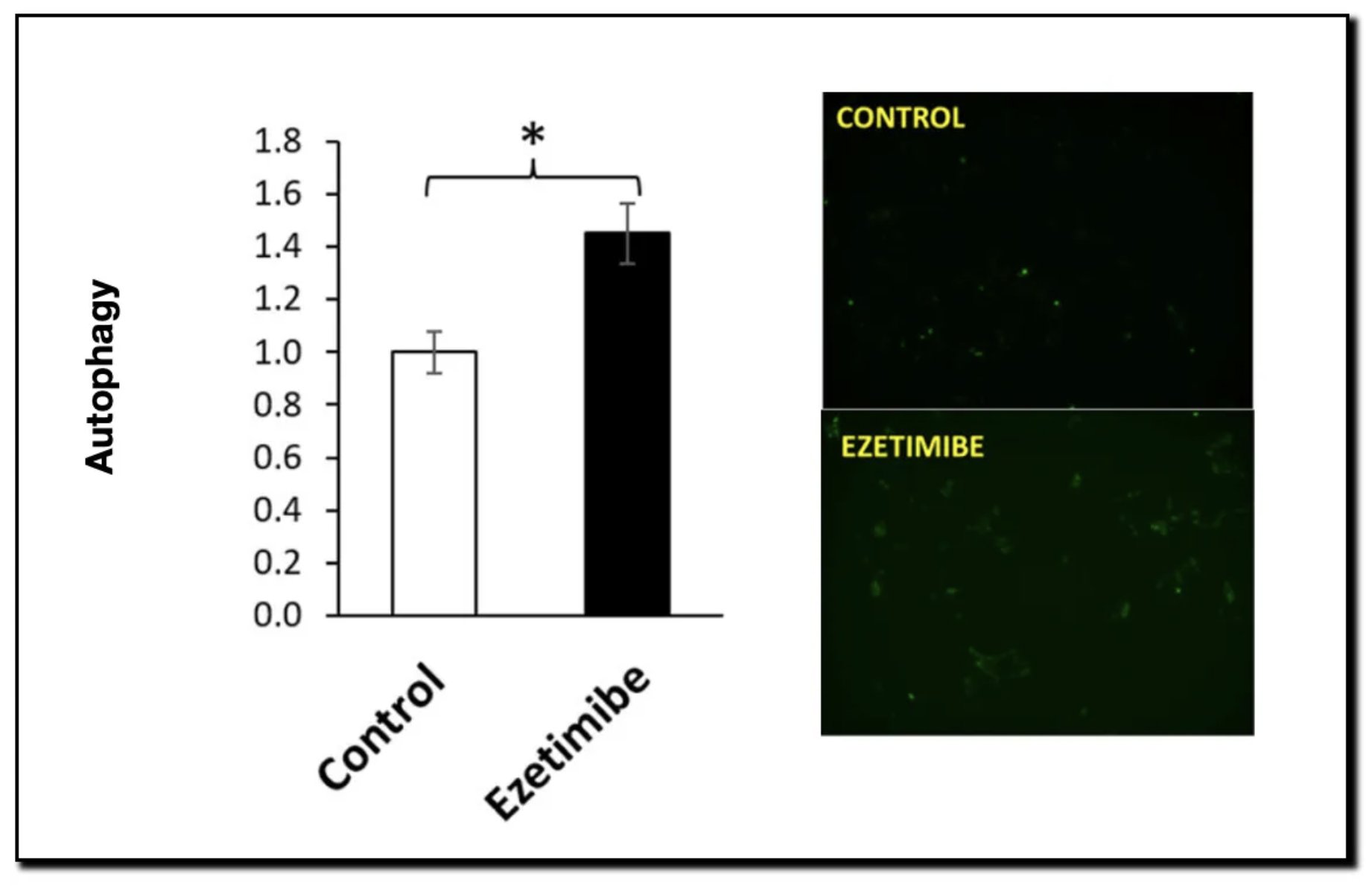
- Result: By preventing this “glue” from forming, aggregates become unstable and are cleared via autophagy. The study confirmed that Ezetimibe treatment significantly upregulated autophagy markers in cell lines.
Novelty
- Mechanism: Identifies a non-lipid mechanism for Ezetimibe. Most literature focuses on NPC1L1 inhibition (cholesterol absorption). This paper posits that Ezetimibe acts as a direct protein-protein interaction (PPI) inhibitor in the brain.
- Biomarker: Proposes the 14-3-3 gamma :: HK1 complex as a specific biomarker for AD and heart-disease-associated neurodegeneration.
- Effect Magnitude: The reported 7-fold risk reduction in the retrospective cohort is significantly higher than existing interventions, though this outlier status requires skeptical verification.
Critical Limitations
- Missing Mammalian In Vivo Data: The study jumps from C. elegans to human retrospective data. There is zero mouse or rat data presented to verify pharmacokinetics, blood-brain barrier (BBB) penetration, or behavioral rescue in a mammalian brain.
- Retrospective Confounders: While propensity matching was used, a 7-fold reduction (RR 0.14) is statistically suspicious for a complex disease like AD. Unobserved variables (e.g., socioeconomic status, frequency of doctor visits, concomitant medication use) likely inflate this number.
- Dosing Translation: The study relies on standard clinical doses of Ezetimibe (10mg/day) in the retrospective data. It is unknown if this standard dose achieves the necessary concentration in the human brain to disrupt the 14-3-3 gamma :: HK1 interaction, as effective brain tissue concentrations were not measured in vivo.
Reasoning Framework: Probabilistic & Bayesian
- Acknowledge Uncertainty: The mechanism is plausible and supported by in vitro data, but the clinical efficacy relies entirely on retrospective data, which is prone to selection bias. The lack of an intermediate mammalian study increases translational risk.
-
Quantify Confidence:
- Mechanism of Action (14-3-3/HK1 blocking): [Confidence: Medium] - Well supported in vitro, but needs in vivo confirmation.
- Clinical Efficacy (Preventative): [Confidence: Low-Medium] - The effect size is too large to be taken at face value without a prospective randomized controlled trial (RCT).
- Safety: [Confidence: High] - Ezetimibe is a widely used, generic drug with a well-understood safety profile.