I think we’ve identified a blood everolimus test above in this thread - please report if you do use it and the cost, and best places to get it.
I found the original paper for the comparison table from that first post:
Long-term outcome of everolimus treatment in transplant patients
Thanks for digging up these resources. From a quick glance, some frustrating inconsistencies.
For what it’s worth, I’ve also tried chewing everolimus and and swishing it in my mouth for up to a minute, and then swallowing it. It doesn’t have an unpleasant taste, and I’ve noticed subjective effects within minutes! I limit this method of administration to no more than 5mg everolimus at the moment however.
Interesting. But i wonder if everolimus is like rapamycin and has to be protected from stomach acid so it gets into the small intestine and absorbed. Chewing could break the barrier that provides the protection against the stomach acid.
The study they cite for this is:
This is an in vitro study testing a standard concentration of each drug on primary human aortic endothelial cells (HAEC) after one-time exposure. As many people will know, rapa does not acutely inhibit mTORC2, but somehow destabilizes it after prolonged exposure: that’s part of the rationale for intermittent dosing. So I don’t think this study should be relied on too much as regards the relative effects of the two drugs on it.
The basis for Dr. Stanfield’s statement that everolimus inhibits mTORC2 more than rapa here is not clear; no citation is given.
On the other hand, the Lamming study found that everolimus is less prone to inhibit mTORC2 after long-term administration is in vivo — at least in the liver. It is in mice — but so are nearly all the other studies on which we’re pinning possible human use. This is the same study cited for saying everolimus is less prone to inhibiting mTORC2 as cited in the Wikipedia article.
I guess chewing and swishing results in something like a sublingual administration which may be the reason for the quick effects. But yeah, i don’t know what happens in the stomach.
Did you ever contact them? What did they say?
I have not yet ordered any everolimus or spoken to Life Extension about the potential for a lower cost version of the everolimus blood test.
Last year when I emailed them about the sirolimus blood test I just contacted them as a concerned customer… so if anyone is interested in a lower cost everolimus blood test i encourage you to contact them and tell them there is a growing interest and demand in this test, just like the blood sirolimus test. Here was my original contact effort towards their lab customer support: How to get a Rapamycin (sirolimus) Blood Level Test
Oh — really? You were indicating you would do so back last summer. What changed your mind or caused you to delay commencement?
I ended up getting into the plasmapheresis clinical trial which lasts until this month - so I’ve paused my rapamycin / everolimus during the trial. will restart next month.
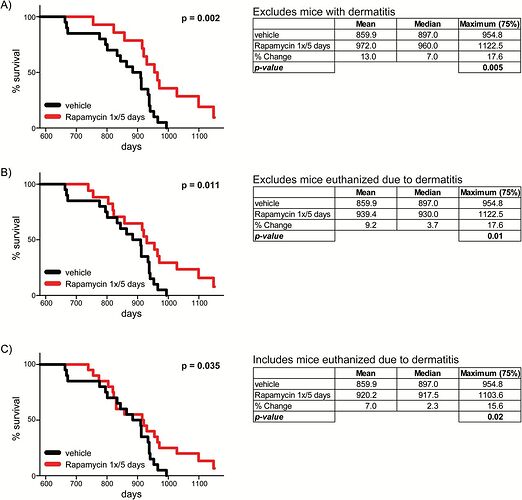
It’s true that the great majority of lifespan studies used continuous dosing. However, there is exactly one mouse study with a protocol similar to the once-weekly-type protocol used in the Lamming 2014 glucose tolerance study (which you quoted from above) and also used in the Mannick everolimus human trial (which is the best available evidence for a potential anti-aging effect in humans):
Intermittent Administration of Rapamycin Extends the Life Span of Female C57BL/6J Mice
Sebastian I. Arriola Apelo, Cassidy P. Pumper, Emma L. Baar, Nicole E. Cummings, Dudley W. Lamming
The Journals of Gerontology: Series A, Volume 71, Issue 7, July 2016, Pages 876–881,
We recently identified an intermittent rapamycin treatment regimen—2mg/kg administered every 5 days—with a reduced impact on glucose homeostasis and the immune system as compared with chronic treatment; however, the ability of this regimen to extend life span has not been determined.
Here, we report for the first time that an intermittent rapamycin treatment regimen starting as late as 20 months of age can extend the life span of female C57BL/6J mice.
A lot of caveats come with that result, the most important of which are:
- these are all female mice (lady-mice do better on rapa than boy-mice), so it might overstate what you would see in males; and
- There were only 20 mice in the vehicle-treated and no more than 20 rapamycin-treated mice (up to 6 rapa mice were excluded in (a) and (b)), which is not nearly enough to give a reliable lifespan result.
Yes - Jane Mannick has said that they just used this protocol and study as a basis for their own human clinical study.
You mean the just-cited lamming study? The first Mannick trial was in 2014, and this study was in 2016. The earlier glucose study by Lamming was in 2014, but it doesn’t seem plausible that they would have turned their doses for a clinical trial around that quickly.
Yes, you caught that critically important distinction: “we report for the first time that an intermittent rapamycin treatment regimen starting as late as 20 months of age can extend the life span of female C57BL/6J mice”. The study makes no reference to male mice.
Chronic rapamycin treatment extends lifespan of both male and female mice. But clearly, from this study, and others, there is sexual dimorphism in some longevity interventions, likely connected to hormonal biology (eg.17α-Estradiol)
That seems an oversimplistic way to do a dose-conversion: they were just measuring how much drug it took to get the same trough blood concentration of either drug. But each drug has different pharmacokiinetics beyond the trough concentration, and also different pharmacodynamics (e.g., differences in mTORC1 vs. mTORC2 inhibition at a given concentration and a given time of exposure) and tissue selectivity (e.g. everolimus has greater access to the brain). So just saying how much of either drug you have to take to get steady-state 12-15ng/mL of each is not likely very meaningful.
Agreed. As the “Everolimus and Sirolimus in Transplantation-Related but Different” paper from 2015 said:
Although binding of everolimus to FKBP-12 is approximately 3-fold weaker than that of sirolimus [19], everolimus blood trough concentrations targeted in transplant patients are typically lower than for sirolimus (3–8 ng/mL versus 4–20 ng/mL [24–26]).
According to Joan Mannick (25:31), everolimus is equivalent to rapamycin.
And that you actually can take a lower dose of everolimus to get the same treatment effect as rapamycin. Meaning you get higher levels at the same dosage.
@RapAdmin what if people have taken twice the dosage of everolimus thinking it’s half as effective but it is actually more potent ![]()
Everolimus seems to be used as a cancer drug rather than for immunosuppression and for organ transplants. Rapamycin doesn’t seem to be used for that, at least much. It can stop the growth of certain cancers.
Brand name Afinitor.
I’m thinking another positive with everolimus, is that the scientists behind it spent a lot of money, effort, and time, to create a rapamycin alternative. That process might’ve been intended to make a better alternative, and thus there is the chance they’ve succeeded.
I would think that the uncertainty with Everolimus is greater than Rapamycin.
Why do you think that? Everolimus has been used in the only clinical trial to show weekly dosing being safe / improving aging (possibly reversing immunosenescence)? It was developed in the 90’s, a looot of clinical trials has used it.