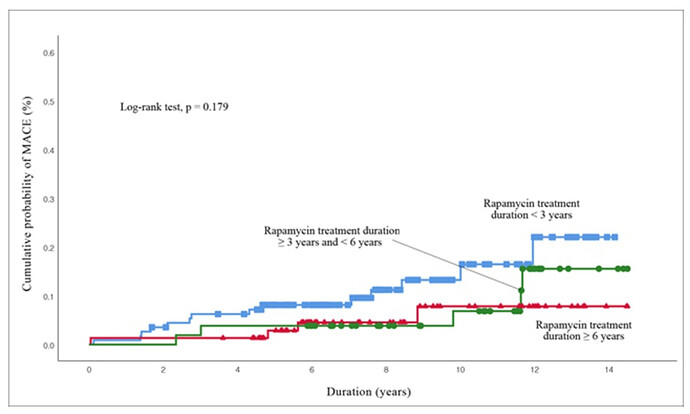
One of the biggest issues with elevated rapamycin doses is side effects, precluding prolonged exploration. We have many long threads on whether some of these perturbations, notably CBC and lipids are benevolent or actually impact other comorbidity pathways (eg. CVD), thus short circuiting the ultimate objective…lifespan extension.
Here’s an amazing paper showing that the initial “short term” dysregulation on rapamycin is actually temporary, and over time, resolves itself. So maybe…we need to ride out the initial shock change to systemic biomarkers and allow the body some time to adjust to this new “hormetic” steady state to reach the ultimate goal…lifespan extension? So instead of bailing, hold our nose and ride it out?
Duration of Rapamycin Treatment Has Differential Effects on Metabolism in Mice
https://sci-hub.se/10.1016/j.cmet.2013.02.008
Beginning at the same age (3 months), male mice were injected i.p. with 4 mg/kg BW rapamycin or vehicle every other day according to the protocol by Chen et al. (2009) and were then sacrificed after 2, 6, or 20 weeks of rapamycin treatment.
Chen 2009 paper (hugely fundamental paper in and of itself showing rapamycin rejuvenating HSC hematopoiesis)
mTOR Regulation and Therapeutic Rejuvenation of Aging Hematopoietic Stem Cells
Results of 6 weeks of rapamycin treatment generally resembled the effects of 2 weeks of
rapamycin treatment but appeared to represent a transitional state with regard to insulin sensitivity and HOMA-IR. In contrast, 20 weeks of rapamycin treatment had very different and generally ‘‘beneficial’’ effects, which may be a mechanism of extended longevity in mice by rapamycin. (20 weeks in mouse time is approx 14 yrs human time)
These data indicate that the actions of mTORC1 and mTORC2 might not be fully separate, and instead it may be their balance that determines the final impact on insulin signalling and longevity
Thus, various measures of insulin signaling and its downstream metabolic effects changed drastically from unfavorable after 2 or 6 weeks of rapamycin treatment toward largely beneficial after 20 weeks of rapamycin treatment. In other words, duration of rapamycin treatment emerges as a key determinant of not only the magnitude but, importantly, also the direction of the responses.
Liver mass was increased after 2 weeks of rapamycin treatment, but it no longer differed from that
of controls after 20 weeks of treatment. Thus, body features associated with metabolic syndrome, including smaller pancreas and enlarged liver, appeared in the mice with 2 weeks of rapamycin treatment, but with continued treatment these features returned to normal levels, and adiposity, body weight, and food consumption were decreased
The most striking differences between the effects of short versus prolonged rapamycin treatment concern insulin signaling, glucose and lipid homeostasis, and metabolism
Unexpectedly, considering the similar blood levels of rapamycin and inhibition of pS6
(Figure 4), prolonged rapamycin treatments in the mice no longer inhibited levels of pmTOR, pS6K1, p4EBP1, or pAKT, while it remained effective in inhibiting pS6. The molecular mechanism for the disparate responses of pS6K1 and pS6 remains unclear.
In the present study, alterations in insulin sensitivity induced by different durations of rapamycin treatment were closely associated with changes of glucose and lipid homeostasis and metabolism, as well as body composition. After 20 weeks of rapamycin treatment, the mice were lean with enhanced insulin sensitivity
By showing differences between metabolic responses to short versus longterm rapamycin treatment, the present findings provide a likely explanation of the paradox of reported detrimental effects of rapamycin on insulin signaling and its ability to extend longevity
Here we show that detrimental effects of rapamycin treatment were only observed during the early stages of treatment. These effects were reversed or diminished in mice treated for 20 weeks, with better metabolic profiles, increased oxygen consumption and ketogenesis, and markedly enhanced insulin sensitivity. Thus, prolonged rapamycin treatment lead to beneficial metabolic alterations, consistent with life extension previously observed. Our findings provide a likely explanation of the ‘‘rapamycin paradox’’ and support the potential causal importance of these metabolic alterations in longevity.
This is profound stuff. Does this translate to humans? Have we seen this in some of the longer term rapamycin clinical trials (years), but perhaps confounded and short circuited in cohorts with severe life limiting comorbidities? If 20 weeks is equivalent to 14 yrs human time…it could take some time to revert, well beyond what many people would be willing to endure?