1.2 The Non-Antimicrobial Pivot: MMP Inhibition
The rationale for “Low-Dose Doxycycline” (LDD) in aging is predicated on the decoupling of its antibiotic and anti-inflammatory activities. Standard antibiotic doses (100–200 mg) achieve serum concentrations sufficient to inhibit bacterial protein synthesis (via the 30S ribosome). However, much lower concentrations are required to inhibit Matrix Metalloproteinases (MMPs), the zinc-dependent endopeptidases responsible for extracellular matrix (ECM) degradation.
Doxycycline inhibits MMPs (specifically MMP-1, MMP-2, MMP-8, MMP-9, and MMP-13) through a distinct mechanism:
-
Cation Chelation: MMPs require Zinc (Zn²⁺) at the catalytic site and Calcium (Ca²⁺) for structural stability. Doxycycline, possessing a phenol-diketone moiety, acts as a chelator, stripping these essential ions and rendering the enzyme inactive.
-
Oxidative Inhibition: The drug can scavenge Reactive Oxygen Species (ROS) such as hypochlorous acid, which activates latent pro-MMPs. By neutralizing the activator, doxycycline prevents the enzymatic cascade of tissue destruction.
-
Transcriptional Modulation: It downregulates the mRNA expression of inflammatory cytokines (IL-1β, TNF-α, IL-6), which are upstream inducers of MMP synthesis.
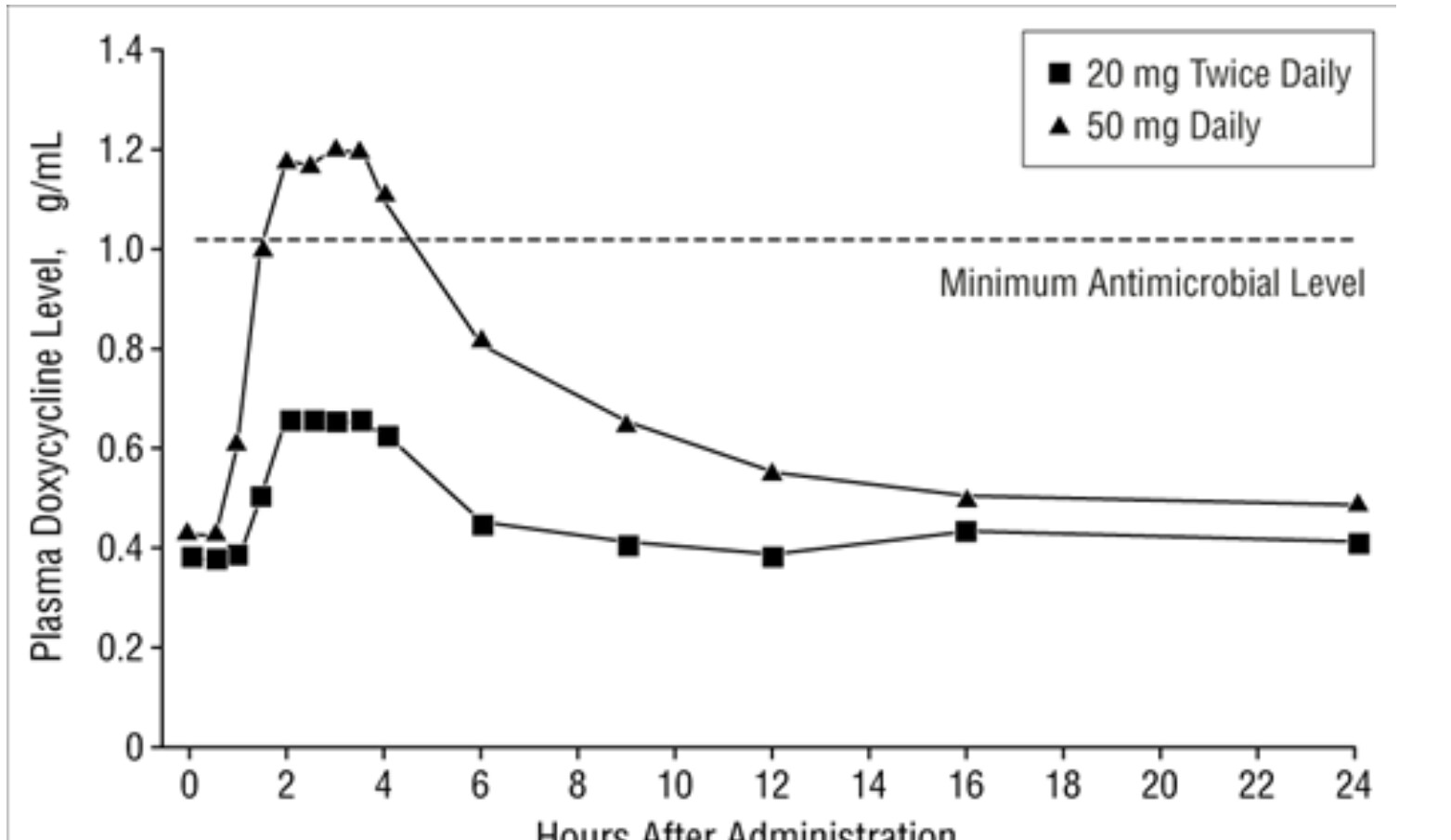
This mechanism led to the development of sub-antimicrobial dose doxycycline (SDD), formulated as 20 mg (Periostat) and later 40 mg controlled-release (Oracea). These formulations maintain serum levels (0.3–0.8 µg/mL) that are potent against MMPs but fall below the minimum inhibitory concentration (MIC) for common pathogens, thereby avoiding the selection of resistant bacterial strains—a crucial safety feature for any proposed “anti-aging” maintenance therapy.
2. The Mitochondrial Mechanism: A Geroscience Auditing
The most profound theoretical basis for doxycycline as a longevity drug lies in the endosymbiotic origin of mitochondria. Because mitochondria evolved from α-proteobacteria, they retain a translational machinery (the mitochondrial ribosome, or mitoribosome) that bears significant structural homology to the bacterial ribosome. Consequently, antibiotics targeting the bacterial ribosome often have “off-target” effects on mitochondrial function. In geroscience, these effects are repurposed as “on-target” mechanisms for mitohormesis.
2.1 Mitoribosomal Inhibition and the UPR^mt
The mammalian mitochondrial ribosome (55S) consists of a small (28S) and a large (39S) subunit. Doxycycline binds reversibly to the 16S rRNA of the bacterial 30S subunit; in eukaryotes, it binds with lower but significant affinity to the mitochondrial 28S subunit.
This binding inhibits the translation of the 13 proteins encoded by mitochondrial DNA (mtDNA), all of which are essential subunits of the oxidative phosphorylation (OXPHOS) complexes (I, III, IV, and V).
-
The Effect: This creates a specific stoichiometric imbalance between mtDNA-encoded proteins (which are reduced) and nuclear-encoded mitochondrial proteins (nDNA, which continue to be synthesized).
-
The Signal: This “mitonuclear protein imbalance” is a potent stress signal. It triggers the Mitochondrial Unfolded Protein Response (UPR^mt). In an attempt to restore homeostasis, the cell upregulates chaperones (e.g., HSP70, HSP60), antioxidant enzymes (SOD2), and metabolic regulators.
-
The Outcome: This is the essence of mitohormesis—the concept that mild mitochondrial stress preconditions the organism to survive longer. Doxycycline-treated C. elegans and plants show induction of stress genes like AOX1A, CPN10X, and BCS1.
2.2 Bioenergetic Reprogramming
The inhibition of mitochondrial translation by doxycycline forces a metabolic shift.
-
Reduced Respiration: Studies in H9C2 cardiomyoblasts and various carcinoma lines demonstrate a dose-dependent reduction in routine respiration, ATP-linked respiration, and maximal uncoupled respiration.
-
Glycolytic Shift: To compensate for the loss of OXPHOS efficiency, cells shift toward aerobic glycolysis (the Warburg effect). This is evidenced by increased glucose consumption and lactate production.
-
ATP Depletion: Depending on the dose, intracellular ATP levels drop. In C. elegans, doxycycline reduced ATP consumption, which correlated with lifespan extension, supporting the “rate of living” theory where reduced metabolic flux decreases damage accumulation. Conversely, Vitamin C (a pro-oxidant) increased ATP levels and failed to extend lifespan, reinforcing the necessity of metabolic suppression for longevity in this model.
2.3 The Toxicity Threshold
A critical finding of this audit is the narrow therapeutic window between mitohormesis and mitochondrial toxicity.
-
Low Dose (Hormetic): Induces UPR^mt, improves stress resistance, extends lifespan (e.g., 13 µM in worms).
-
High Dose (Toxic): Causes severe energetic crisis, membrane potential depolarization, oxidative stress, and cell cycle arrest.
-
Evidence: In human glioma cells, high concentrations (>10 µg/mL) led to severe mitonuclear imbalance and growth arrest, whereas lower doses (0.01–1 µg/mL) altered metabolism without cytotoxicity. This implies that for human healthspan, the dosage must be carefully calibrated to avoid inducing a “drug-induced mitochondrial disease”.
5. Clinical Audit: The Human Data
The translation of doxycycline from preclinical models to human geroscience has been mixed. While established in dermatology, its application in complex age-related diseases reveals the limitations of monotherapy and the importance of timing.
5.1 Dermatology and Wound Healing
The use of sub-antimicrobial dose doxycycline (SDD) in periodontitis and rosacea serves as the regulatory and safety anchor for geroscience applications.
-
Oracea (40 mg): FDA-approved for inflammatory rosacea. Studies show it significantly reduces inflammatory lesions and cytokines (IL-1β, MMP-9) without altering the antibiotic susceptibility of the skin or gut flora over long-term use (9–18 months).
-
Collagen & Scarring: Doxycycline’s MMP inhibition has profound effects on dermal remodeling. In models of skin scarring, local doxycycline significantly reduced scar thickness and improved collagen architecture (increasing fiber randomness to mimic natural skin) by suppressing “profibrotic” engrailed-1 positive fibroblasts.
-
Wound Healing Dynamics: There is a trade-off between speed and quality. While some models show it accelerates corneal wound healing, others suggest pan-MMP inhibition may delay initial wound closure in chronic settings. However, for hyper-inflammatory wounds or preventing hypertrophic scars, its ability to modulate the ECM is clinically valuable.
5.2 Neurological Protection: The Blood-Brain Barrier
Doxycycline readily crosses the Blood-Brain Barrier (BBB), a property that distinguishes it from many other tetracyclines.
-
BBB Preservation: In models of stroke (MCAO) and traumatic brain injury, doxycycline significantly reduced BBB leakage and cerebral infarct volume. Mechanism involves the upregulation of tight junction proteins (Claudin-5, Occludin, ZO-1) and the inhibition of MMP-9, which digests the basil lamina of the BBB during ischemic stress.
-
Antioxidant Neuroprotection: Beyond MMP inhibition, doxycycline acts as a direct antioxidant in the CNS, scavenging free radicals and reducing oxidative stress markers (like lipid peroxidation) in brain tissue, offering a second layer of neuroprotection distinct from its antibiotic activity.
5.3 Rheumatology and Tendon Health
-
Osteoarthritis (OA): A large, 30-month randomized controlled trial in obese women with knee OA compared doxycycline (100 mg bid) to placebo. It significantly slowed the rate of joint space narrowing (JSN) by 33–40%, confirming its activity as a Disease-Modifying Osteoarthritis Drug (DMOAD). However, it failed to significantly reduce joint pain, highlighting a dissociation between structural preservation and symptomatic relief.
-
Tendon Healing: Doxycycline has shown promise in improving tendon repair quality. In rat Achilles tendon transaction models, doxycycline treatment increased suture pull-out strength and improved collagen organization at the repair site. Nanofibrous membranes eluting doxycycline have also been shown to significantly improve tendon healing strength at 3 and 6 weeks post-surgery.
5.4 Metabolic Syndrome & Diabetes
Human clinical data supports the preclinical metabolic findings.
-
HbA1c Reduction: A pilot clinical trial in patients with Type 2 diabetes and chronic periodontitis found that low-dose doxycycline (SDD) for 3 months significantly reduced Hemoglobin A1c (HbA1c) levels by 0.9% compared to baseline, whereas placebo groups showed no improvement.
-
Mechanism: This effect is likely mediated by the reduction of systemic inflammation (TNF-α, CRP), which is a known driver of insulin resistance.
5.5 Cardiology: The Aneurysm Disappointment
Given the dramatic survival extension in Marfan mice, doxycycline was tested for preventing Abdominal Aortic Aneurysm (AAA) expansion.
-
The Trial (N-TA3CT): A multicenter trial randomized patients with small AAAs to doxycycline (100 mg bid) or placebo.
-
The Result: No difference in aneurysm growth rate at 2 years (0.36 cm in both groups).
-
Analysis: While doxycycline reduced plasma MMP-9, it failed to arrest the biomechanical expansion of the aorta. Furthermore, some evidence suggests that in aortic smooth muscle cells, doxycycline may induce mitochondrial dysfunction and endoplasmic reticulum (ER) stress, potentially counteracting its matrix-preserving benefits in this specific tissue context.