Check the other thread with the genetic studies and clinical trials.
You’re right that the dose used is crazy high (7 g/kg in mice, equivalent to ~40 g/day for a human?). We need the full paper to understand why they chose that dose.
Hi adssx, I’m around at 80 kg so x 7 g per kilogram would be 560 g per day without allometric scaling, but even with, the dose would be crazy high. Most people supplement with just a few grams per day.
It’s always a good idea to read the full article before drawing conclusions, but I’m not sure I’m going to take the time for this one. ![]()
I could be wrong, but I think you’re reading too much into this. They’re talking about a reduced rate of uptake of an incremental labeled dose of DHA after long-term high-dose supplementation. Note the Figure caption: “Images are from the 10-30 min window post injection of the tracer”. It wouldn’t be surprising if the brain (either the tissue or the BBB) more avidly took up or incorporated incremental DHA when levels are low or physiological, and it took up or incorporated DHA more slowly when highly replete. It would kinda be problematic if it wasn’t.
So … taking fish in the diet, before Alzheimer’s Changes occur seems to rate reduce those with ApoE4’s (and presumably even better in those without ApoE4’s) getting AD. There is also a solid relationship in those without ApoE4’s. The issue is, once there is MCI or AD, I don’t think we have any evidence of benefit.
From my perspective, the issue with supplements, unless they have phospholipids, or perhaps if money is no object plasminogens, is an issue of brain penetrance, especially in those with ApoE4s.
Pushing levels too high isn’t a great choice and in general I don’t recommend over 1000 mg/day total. The strategy of pushing EPA has some validity, but overall, my assessment - at least how I’m clinically implementing is to favor having fish, and supplement with Omega 3’s with a decent mix of DHA/EPA with phospholipids or plasminogens.
- A dose-response meta-analysis of observational studies I in Nutrition Reviews found that up to 2 portions of fish per week reduced Alzheimer’s disease (AD) risk by 30% (RR: 0.54–0.89; N = 3 studies) and all-cause dementia by 10% (RR: 0.79–1.02; N = 5 studies) 1.
- A cross-sectional analysis in JAMA II of 286 autopsied brains showed that seafood consumption (≥1 meal/week) was significantly correlated with less AD pathology in APOE4 carriers, including lower neuritic plaques (β = –0.69; 95% CI: –1.34 to –0.04) and neurofibrillary tangles (β = –0.77; 95% CI: –1.52 to –0.02) 2.
@CronosTempi, any thoughts on daily consumption of High DHA fish oil (with DHA 500mg + EPA 100mg) x 2 = Daily total of 1,000mg DHA + 200mg EPA ,… In reference to the cited article, is this a net negative ?
Daily DHA seems a net negative (but based on more than a single paper). Pulsatile, like for example once weekly, not enough data. The issue is that while DHA is vital, there are problems with supplementing - it somehow interferes with endogenous conversion, so you end up with less available - very paradoxical (I posted a paper to that effect). So it’s supplementing that’s problematic. There are claims that certain forms of DHA are OK to supplement, but I don’t see that as proven. I personally stick to modest EPA and a lot of dietary ALA. I also consume fish twice weekly. There may be an issue with age, conversion becoming less efficient, but that’s getting into the weeds.
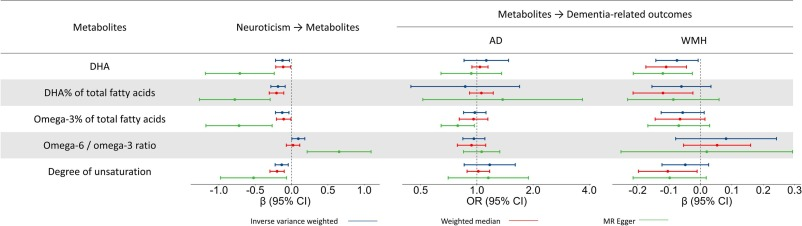
Neuroticism, omega-3 fatty acids, and risk of incident dementia 2025
Omega-3 fatty acids, especially docosahexaenoic acid (DHA), were inversely associated with both neuroticism and dementia risk.
Causal relationships were confirmed, showing that neuroticism leads to lower DHA levels, which in turn contribute to greater cerebrovascular burden.
Mendelian randomization analysis suggested that high levels of neuroticism reduce DHA levels, which, in turn, contribute to white matter pathology, a hallmark of VaD.
So cognitive issues cause low DHA rather than the other way around?
OK, let’s assume so. However, I would highlight another sentence from your quote:
“Mendelian randomization analysis suggested that high levels of neuroticism reduce DHA levels, which, in turn, contribute to white matter pathology, a hallmark of VaD.”
Neuroticism —-> Low DHA —-> VaD.
In this scenario, the problem with “neuroticism” is that it leads to “low DHA”, and if “low DHA” leads to, i.e. is causative (rather than just associated with) per the passage you quoted and I highlighted, of Vascular Dementia, then if we can prevent the lowering of DHA, then assuming this is the causative mechanism, we should be able to prevent the white matter pathology that’s a hallmark of VaD.
Neuroticism can be characterized as “cognitive issues” as you have done in your statement I quoted first. But note, neuroticism in and of itself is not diminishment of intellectual ability like in dementia, but more along the lines of a personality disorder. If you can abolish the downstream effect of prolonged neuroticism (in this scenario) resulting in the lowering of DHA, then prolonged neuroticism should not result in white matter pathology development in vascular dementia and will just remain a personality disorder.
That would argue for finding a means of preventing the decline of DHA (which according to the mechanism that paper outlined) leads to VaD downstream from neuroticism.
But whether supplementing with DHA in fishoil form is the optimal way of raising DHA levels in the brain (or other tissues) is a whole different question - and I have previously posted the paper showing the paradoxical impact of exogenous DHA on the endogenous status of DHA. Yes, DHA in the brain (and other tissues) is important, but that doesn’t mean you can just supplement with DHA in pills and have that translate to proper incorporation into brain tissue.
So even if “low DHA” —-> “VaD”, doesn’t mean “supplemental DHA pills” —-> “adequate DHA levels”. IOW, a neurotic person supplementing with DHA pills might not prevent the development of VaD.
Bottom line, the story of DHA function and levels in the brain (and other tissues), must be considered within the context of “and how exactly do we control those levels”, because it’s not simply by supplementing with DHA pills. We can also ask “does serum DHA translate into DHA incorporation in brain (and other) tissue”? Because maybe supplemental DHA raises serum levels while suppressing incorporation into tissue. Endogenous DHA conversion incorporates DHA into tissues (including brain) - we know this, because even people who don’t consume any long chain marine (or animal) omega-3 FA, don’t lack DHA in their tissues - if they did lack, then all vegans would be dead because omega-3 FA are essential FA. And we have lifelong vegans in many cultures on a multigenerational scale. They do it by converting short chain ALA n-3 to DHA. Studies show that vegans DHA status is just as high (and according to many studies higher!) than omnivores. And again as in the paper I posted previously, supplemental exogenous DHA interferes with that conversion, thus being a net negative. It is therefore crucial to make a distinction between the importance of “tissue DHA” status and “supplemental exogenous DHA”.
Not exactly:
We used WMH as a proxy for VaD due to the lack of genome-wide association studies (GWAS) data and valid genetic instruments for VaD (Ikram et al., 2017).
MR analyses showed that neuroticism causally linked to lower omega-3 fatty acid levels (DHA, DHA% and omega-3 % of total fatty acids) and the degree of unsaturation. Neuroticism also causally increased the omega-6/omega-3 ratio. Further, DHA causally linked to lower WMH volume (Fig. 3; details in eTable 9). Other metabolites showed consistent directional associations with WMH volume and neuroticism, although these associations were not statistically significant. No causal links were found between any metabolites and AD.
GWAS of VaD are generally underpowered and use heterogeneous diagnostic criteria, resulting in a lack of reliable genetic instruments for MR analysis (Ikram et al., 2017).
So:
Neuroticism → Lower DHA → Higher WMH volume
But low DHA does not cause AD and we don’t know if low DHA causes VaD or not.
Actually, doesn’t the chart imply that high DHA is linked to lower WMH volume? [Yes: edited]
You mean the forest plot? I see lower DHA (shifted to the left) and corresponding lower WMH (shifted to the left). And higher AD (shifted to the right).
But AD is Alzheimer’s disease, a specific form of dementia. VaD is vascular dementia without the hallmarks of Alzheimer’s.
By the way, interesting epidemiological factoid: Japan has much higher levels of seafood consumption (compared to the Western diets). Fish are high in DHA. Japan has low levels of AD, but high levels of VaD. High DHA, but high VaD? Of course these are just population level epidemiological studies, but still somewhat interesting. After all, Japan also has higher smoking rates and smoking is associated with dementia.
No, it’s high DHA => lower WMH volume, which is good as white-matter hyperintensities are considered bad (that confused me). I’ll edit my message above.
No: two MR methods are a bit shifted to the right, while one is shifted to the left. None are statistically significant, though.
(And I know what VaD is ![]() )
)
So, for the people in the cheap seats, are we still off DHA? ![]()
I’m taking it 1-2x per week as a vegan to emulate me being a fish eating person (thank you cronos). EPA on most other days.
I don’t use DHA. I’ll change my mind when good data in favor of it appears (RCT, MR, or longitudinal study adjusted for supplementation, fish consumption, exercise and income).
For clarity, I’m in no way saying she is correct, but I’m simply sharing what just arrived in my inbox from gethealthspan.com
I think you’ve mentioned her before, so this is probably not new to you
Also @CronosTempi
The link you shared says “EMAIL_CAMPAIGN_2023_08_10_04_00” so it seems to be an old campaign. We’ve already discussed this article. Rhonda Patrick is a bullshiter and what she says is wrong, neither lysophosphatidylcholine-bound omega-3 fatty acids nor krill oil increase brain DHA in APOE4 mice (and she never answered my emails and tweets about these papers): Predicting Alzheimers & Dementia (and minimizing risk) - #554 by adssx
Also in supplements the DHA can oxidise faster potentially compared to EPA in regular ethyl ester form. The EPA only versions are too expensive for most to run long term at a good enough dose. Though it doesnt seem that the EPA+DHA combinations (as long as EPA>DHA in them) will have as negative effect as DHA only
I wouldn’t take RP’s advice for a variety of reasons. That said, I have a slightly different view than Antoine. The fact is, we don’t have data on supplementing a modest amount of DHA in a pulsatile way, like once a week (similar to fish eating). After all, it’s the dose that makes the poison, and hormesis is a perfect example. What if the same is true for DHA? Now, I never supplemented with DHA. But if I were an ApoE4 with family history, I would supplement with EPA and I would take a once weekly small additional DHA dose. This would be based on risk/reward odds distribution of possible harm vs covering options. It’s insurance against being wrong, essentially game theory, built in fault tolerance.
EDIT: I have glanced over that RP text, but there’s a lot of nuance to discuss, and I can’t do it right now. However, there are several assumptions built in I disagree with.
Emerging evidence suggests that omega-3 fatty acids, particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), may provide neuroprotective benefits in individuals with cognitive impairment and dementia. These fatty acids are hypothesized to influence brain function through anti-inflammatory, antioxidative, and membrane-stabilizing effects. Resting-state functional MRI (rs-fMRI) offers a non-invasive approach to investigate spontaneous brain activity, and the amplitude of low-frequency fluctuations (ALFF) has been validated as a robust metric for assessing neural activity.
This study investigates whether a 24-month intervention with EPA, DHA, or their combination can modulate hippocampal ALFF in individuals with mild cognitive impairment (MCI) or dementia.
Participants (n=59) were randomized into four groups: (1) EPA 0.8 g/day + DHA 0.35 g/day (n=13), (2) EPA 0.8 g/day (n=15), (3) DHA 0.35 g/day (n=17), and (4) placebo (n=14).
These findings suggest that 24-month supplementation with EPA, DHA, or their combination does not significantly influence hippocampal ALFF in individuals with MCI or dementia. This absence of modulation implies that the neuroprotective effects of omega-3 fatty acids may not operate through alterations in spontaneous neuroactivity of the hippocampus, as measured by ALFF. Interestingly, the observed positive correlations between increasing ALFF and higher ADAS-cog scores, which indicate more profound cognitive impairment, highlight a potential compensatory mechanism, where heightened spontaneous neuroactivity in the hippocampus may reflect the brain’s attempt to counteract cognitive decline. This compensatory process aligns with prior studies suggesting that increased neural activity in the hippocampus may be a response to pathological stress, such as beta-amyloid accumulation or synaptic dysfunction. Despite these intriguing correlations, the lack of significant group effects underscores the need for further research to explore other neural mechanisms by which omega-3 fatty acids may exert neuroprotective effects. Future studies should also investigate whether specific subpopulations or alternative biomarkers may demonstrate differential responses to omega-3 supplementation.