No. And the study you quoted is irrelevant—no one here is suggesting extended-release Niacin with Laropiprant. Immediate-release Niacin has a different safety profile, and that’s what’s being discussed.
relaxedmeatball - “I would also like to see the data about MACE reductions. I much prefer those hard outcome data, rather than just chasing after biomarker numbers. As somebody said earlier, chasing improvements in HDL-C as “cardioprotective” showed the futility of that approach.”
Lp(a) isn’t just a biomarker—it’s a causal driver of cardiovascular disease. Reducing it reduces risk. Period. We don’t yet have outcome trials proving that lowering Lp(a) reduces MACE, but that’s because those trials take time, not because the causal link is in doubt. I agree that waiting for new therapies makes sense if you’re comfortable with your current risk, but for those with very high Lp(a) and few options, it’s reasonable to act now.
Sorry if I upset you
First - I am not saying that niacin does help people with high Lp(a). I am just saying that you can not confidently say that it does not (without putting forward other arguments that you did).
The statistics do not work the way you are trial to use the non Lp(a) focused CVD trials
- as you know I said it’s very likely that there is a net negative outcome for people who are not high Lp(a) of taking niacin (and it seems like you believe that is what the data says also)
- but the only way your reasoning works for inferring that there is not a net positive effect on high Lp(a) people from niacin is to know that the effect on the low Lp(a) people is not that negative.
Because if that effect of niacin is even half as negative on the low Lp(a) participants (that are more than twice as many in the trial) as the effect of niacin is positive for the high Lp(a) participants then trial results will be consistent with a net negative result.
So it sounds like the correct answer based on the information in your posts and those trials is that we still don’t know
…and not that one should confidently rule out taking it
I personally do not take niacin even if I don’t have ideal Lp(a)
- but it seems like a quite close call too me and not something where the answer is that it was as clear “bust”
So look at the niacin trials without laropiprant or delayed release, it doesn’t change my point to look at the trials.
Wondering what about natural PCSK9 inhibitors? No one talking on that? Melatonin, fistein, berbarine and bergamot together (my GP suggested that) etc etc.
I’d take the same line I always do; that I would much prefer a drug to a supplement, where the choice exists.
Drugs have big clinical trials, control groups, an independent 3rd party to monitor safety and efficacy. Then they get FDA approval, and safety data is still collected (post market surveillance).
Supplements rarely have good quality studies, and they’re usually small and short-term, sponsored by the people selling the supplement etc.
However, my biggest issue is that we no real idea about quality control, purity, truthfulness etc. Time and time again, we see supplements where the dose is nowhere near what’s on the bottle. Sometimes the supplement in question is completely absent. And I’d be nervous about any of those plant extracts because you have no idea the original source quality (laden with pesticides etc) what sort of contaminants (solvent etc) are left behind from the processing. And the manufacturers in general are factories in China, and I don’t trust them that the bag of tea leaves for the bergamot hasn’t been sitting around the warehouse for ages, accumulating rat and cockroach droppings.
So if my idea is to target PCSK9i, I’d choose one of the two monoclonal antibodies, or the siRNA drug, which are much better understood and the quality control is good.
It’s not to say I don’t take supplements at all. But if the choice exists, it’s a pharma product every time.
And what are your thoughts on Co Q10? Cardiovascular Health and Lipoprotein(a): How to Address a Genetic Issue and Support Cardiovascular Health Naturally - TOCOTRIENOL Tocotrienol.org ‘Coenzyme Q10, commonly known as CoQ10, is an antioxidant well known for its cardiovascular health supporting benefits. CoQ10 significantly reduces serum levels of lipoprotein(a), with reductions of 31% compared to 8.2% with placebo in one randomized double-blind placebo controlled trial.[xi] CoQ10 has been shown in meta-analysis to improve endothelial function,[xii] and in other studies to reduce LDL oxidation,[xiii] as well as systolic and diastolic blood pressure in patients with diabetes.[xiv] With the use of statin medications, which inhibit an enzyme known as HMG-CoA reductase, it is very important to include CoQ10 as a supplemental therapy as this enzyme is also necessary for production of CoQ10.’
I read this re niacin today - I think genotypes make a difference: Lipoprotein a: Genes and Lp(a)
Thanks. Yes, we discuss that a bit here and in some of the downstream posts:
@CronosTempi this might be another reason the general trials do not pick up the signal
Not only is high Lp(a) just a minority of people in those trials, but it’s then quite likely it’s mostly (or only) a subset of those with high Lp(a) that have those specific genetics that super respond and/or net benefit. Or what do you think of that part?
The link mentioned genotype → Lp(a) ![]() → ASCVD risk
→ ASCVD risk ![]()
Why not just measure Lp(a)?
Hi @KiwiGuy Yes I think you’re right. It’s difficult to imagine where Lp(a) is successfully lowered but it doesn’t translate to better clinical outcomes.
Luckily quite a lot of drugs are on the way. There are three drugs in phase 3:
OCEAN(a) trial - Olpasiran, siRNA with injection every 12 weeks, 70-100% lowering
HORIZON trial - Pelacarsen, anti-sense oligo with injection every 1-4 weeks, 70-100% lowering
ACCLAIM-Lp(a) trial - Lepodisirin, siRNA with injection every 6 months. Reduced Lp(a) 97%
And two more drugs in phase 2:
KRAKEN trial - Muvalapin, a small molecule, once daily oral administration, 86% reduction
SLN360, siRNA, injection every 4-6 months, 85.6% reduction
So very soon we should have more than one option
Any sense when the first one will file with the FDA?
No, sorry. But my cardiologist said he “should be able to prescribe something within 2025”, so presumably not very long.
That’s really interesting, and I hadn’t heard of a link between CoQ10 and Lp(a) before.
I found the original study (Serum concentration of lipoprotein(a) decreases on treatment with hydrosoluble coenzyme Q10 in patients with coronary artery disease: discovery of a new role - PubMed) and it’s quite small, and pretty old (1999).
I found a 2016 analysis which pooled together several trials (Supplementation with coenzyme Q10 reduces plasma lipoprotein(a) concentrations but not other lipid indices: A systematic review and meta-analysis - PubMed) They found a small decrease in Lp(a) with CoQ-10 treatment, but no dose-response relationship. The decrease in Lp(a) was larger if the patients had a higher Lp(a) level to begin with. To me, this looks like a phenomenon we call “regression to the mean” where outliers tend to move towards the average over time. I.e. if somebody has high Lp(a) it’s more likely to go down than to go up.
Honestly, I think the evidence is not very strong. There are a few supplement company-sponsored trials, a study out of India, another out of Iran. Just not the most reliable type of evidence. I think CoQ-10 is safe, and not too expensive, so maybe worth a try. But I don’t think it’s going to move the needle.
If you look at my previous post in this topic, there are 5 drugs right now in trials, where an injection every few months is lowering Lp(a) by 80% or greater. That’s what could make a big difference to the thing we care about, which isn’t the Lp(a) number itself, but rather the progression of atherosclerosis.
I am on a forum where some are combining Cavadex with nattokinase. However I am not convinced. Thanks for your input (love your nametag!). BTW read this article re keto and Lp(a) Does a ketogenic diet lower a very high Lp(a)? A striking experiment in a male physician - PMC
Another example that triangulates
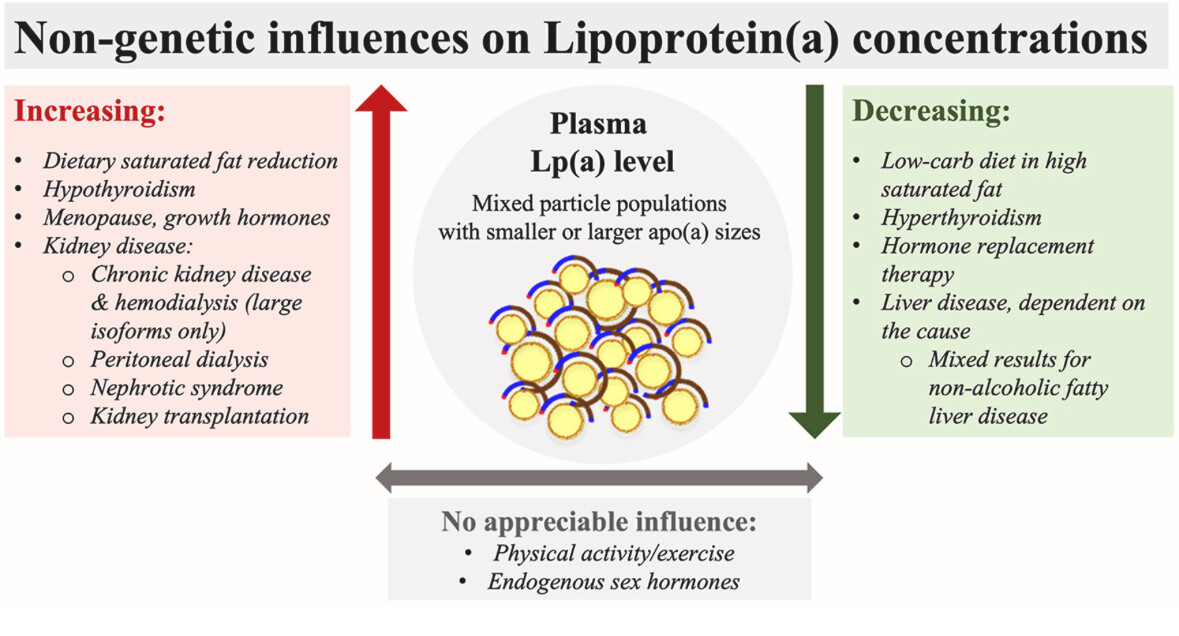
The DELTA (Dietary Effects on Lipoproteins and Thrombogenic Activity) trials observed that reducing saturated fat, replaced by complex carbohydrates, resulted in approximately a 15% increase in Lp(a) levels.
https://www.atherosclerosis-journal.com/article/S0021-9150(22)00183-6/fulltext
@Davin8r - did you ever look into diet impact?
Pelarcarsen was the only one I can find that might have had a chance for approval this year, but phase 3 completion was pushed out to 2026.
Olpasiran phase 3 completion - 12/26, so nothing until 2027 at the earliest
Lepodisirin phase 3 runs until 2029
So, no.
@AnUser et al
I found:
obicetrapib could potentially receive its first regulatory approval in late 2025 or early 2026
It’s not “for” Lp(a, but in the trials lowered Lp(a) by about 50%.
@adssx - you have any more sense on when it may be approved in Europe?
Okay, but how does that affect apoB, how does the total risk change? An increase in Lp(a) can be offset by a much larger decrease in apoB, maybe if it’s six times the absolute number.
Not in the US, unless they radically change plans. US availability is targeted for 2027, EU 2026.
You should listen to recent tech conference presentations hosted on the nams website.