Published: 11 May 2023
Long-term effects of canagliflozin treatment on the skeleton of aged UM-HET3 mice
Gozde Yildirim, Edmara T. P. Bergamo, Sher Bahadur Poudel, Ryan R. Ruff, Manisha Dixit, Bin Hu, Dindo Q. Mijares, Lukasz Witek, Carolyn Chlebek, David E. Harrison, Randy Strong, Richard A. Miller, Warren Ladiges, Timothy G. Bromage, Clifford J. Rosen & Shoshana Yakar
GeroScience (2023)Despite the robust metabolic benefits, reductions in bone mineral density (BMD) and cortical fractures were reported for CANA-treated subjects. … [E]arly clinical studies reported adverse effects on bone, specifically an increased risk of bone fractures, particularly those associated with CANA [6, 8,9,10]. The majority of patients who presented with cortical bone fractures experienced traumatic falls, which may have resulted from postural hypotension [6]. A smaller fraction of SGLT2i-treated patients presented with a small (1.2%), but significant site-specific (total hip) reduction in bone mineral density (BMD) that is expected to lead to an increase in fracture risk [11]. In the CANVAS study [12], 4.9% of the participants experienced a fracture event during follow-up, of which 49.4% were women. One possible cause for loss of BMD could be attributed to the weight loss following treatment. Indeed, weight loss was shown to correlate with increased collagen type 1 β-carboxy-telopeptide, a measure of bone resorption and could explain 3% of fractures [11]. It was these observations that led the FDA to issue a warning about an increased risk of fractures with SGLT2i treatment. However, it should be noted that the mechanisms underlying bone loss with weight loss therapies are not well delineated, and, in some studies, have been shown to be independent of the magnitude of weight loss. …
In collaboration with the National Institute on Aging (NIA)–sponsored Interventions Testing Program (ITP), we tested skeletal integrity of UM-HET3 mice fed control (137 mice) or CANA-containing diet (180 ppm, 156 mice) from 7 to 22 months of age.
Micro-computed tomography (micro-CT) revealed that CANA treatment caused significant thinning of the femur mid-diaphyseal cortex in both male and female mice, did not affect trabecular bone architecture in the distal femur or the lumbar vertebra-5 in male mice, but was associated with thinning of the trabeculae at the distal femur in CANA-treated female mice.
In male mice, CANA treatment is associated with significant reductions in cortical bone volumetric BMD by micro-CT, and by quantitative backscattered scanning electron microscopy. Raman microspectroscopy, taken at the femur mid-diaphyseal posterior cortex, showed significant reductions in the mineral/matrix ratio and an increased carbonate/phosphate ratio in CANA-treated male mice. These data were supported by thermogravimetric assay (TGA) showing significantly decreased mineral and increased carbonate content in CANA-treated male mice.
Finally, the sintered remains of TGA were subjected to X-ray diffraction and showed significantly higher fraction of whitlockite, a calcium orthophosphate mineral, which has higher resorbability than hydroxyapatite.
Overall, long-term CANA treatment compromised bone morphology and mineral composition of bones, which likely contribute to increased fracture risk seen with this drug.
Canagliflozin Compromizes Bone Morphology and Density and Increases Fracture Risk In Mice and Humans
Well, that’s certainly not good news. I wonder how many of the other 'flozins might be suspect.
Darn it. I was hoping to start canagliflozin next. I guess I will stick with Rapa, acarbose and Metformin for prescription meds. Thanks for this.
It’s okay in my opinion because as a non-type II diabetic that takes metformin, I found that adding canagliflozin had no detectable effect on my blood sugar. I think the acarbose only taken before a high-carb meal is the way to go.
It’s probably worth digging into a bit more. As a first pass, I looked up two studies on humans. The CANVAS study, where they observed the 4.9% difference in fracture risk concludes
In conclusion, we could find no clear explanation for either the fracture risk observed in the CANVAS Program or the difference in fracture risk observed in CANVAS compared with CANVAS-R. In practice, however, most fractures are caused by falls and the observed association is likely a consequence of either an unidentified fall-related mechanism or else a chance finding. The null finding for fracture risk with canagliflozin in the recently completed CREDENCE trial has provided important additional insight and somewhat increases the likelihood that the adverse effect observed in CANVAS was a spurious finding.
The CREDENCE study did not see this difference
Rates of fracture were also similar in the two groups (hazard ratio, 0.98; 95% CI, 0.70 to 1.37).
CANVAS used both 300 mg and 100 mg doses, but says that there was no evidence for difference of fracture risk at the two doses. CREDENCE used 100 mg dose of canagaflozin.
CREDENCE link:
The difference is that canagliflozin is a plausible anti-aging agent, whereas metformin is not.
Have you ever tried cana when not taking metformin? Also, when you say it had no detectable effect on your blood sugar, how were you measuring that? As a non-type II diabetic that takes metformin, the only place you’d probably expect to see a difference is on postprandial glucose on CGM or OGTT. Did you try that? You might expect a slight effect on HbA1c, but it might be small and take the entire month to see — again, did you try that?
The data and safety committee,145 which published the higher fracture incidence in the canagliflozin group of the CANagliflozin cardioVascular Assessment Study (CANVAS) in 2013, sparked concern about the effect of SGLT2 inhibitors on bone health and fracture. Watts et al.146 analyzed data from CANVAS and eight pooled non-CANVAS studies and reported the increased incidence of fractures with canagliflozin (4.0%) versus placebo (2.6%) in CANVAS study, while the fracture risk was not observed in canagliflozin treatment in non-CANVAS studies. A subsequent study by Neal et al.,147 which included 10,142 participants with type 2 diabetes and high cardiovascular risk from the pooled data of CANVAS and CANVAS-R trials to assess cardiovascular, renal, and safety outcome found an increased rate of all fractures (15.4 versus 11.9 per 1000 patient-years; HR 1.26, 95% CI 1.04–1.52) in CANVAS but not in CANVAS-Renal (HR 0.76, 95% CI 0.52–1.12).
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial, a RCT of type 2 diabetes with kidney disease found no increased fracture risk in canagliflozin group.149 As a result, the finding of fracture risk in CANVAS likely appeared to be a chance observation, though the possibility remained whether this medication may have exerted its fracture risk through other mechanisms, such as increased risk of falls due to limited data.148
Other studies of different SGLT2 inhibitors on cardiovascular outcome found no evidence of increased fracture between treatments and placebo groups
Although the effect of SGLT2 inhibitors on fracture risk remains controversial, the potential mechanism of SGLT2 inhibitors on bone metabolism is thought to be through changes in calcium and phosphate metabolism. Data suggest that SGLT2 inhibitors increase tubular phosphate reabsorption and lead to secondary hyperparathyroidism and increased fibroblast growth factor-23, resulting in enhanced bone resorption.142 Canagliflozin was found to increase bone resorption markers at 6 and 12 months of treatment, which was not observed for other SGLT2 inhibitors (dapagliflozin, ertugliflozin).143,144,155 A small reduction in bone density at the total femur was observed in the canagliflozin group compared to placebo in a double-blind, placebo-controlled, 2-year trial of over 700 patients with type 2 diabetes.143
To date, there has been limited data of SGLT2 inhibitors on fracture risk. The majority of the evidence came from the CANVAS study, while other studies found no increased fracture risk associated with SGLT2 inhibitors. Because this is a relatively new anti-diabetic agent, and most of the clinical trials and observational cohort studies had relatively short treatment duration (between 1–3 years), fracture risk or bone density was not the primary endpoint of the cardiovascular safety trial, and the assessment of fracture risk, which could take a longer period that might not be apparent during the study period. On the bone effect of SGLT2 inhibitors, cautious data interpretation and post-market long-term safety data research are required.
https://journals.sagepub.com/doi/10.1177/17455057231165549
This is somewhat reassuring, but the parts that I emphasized suggests some reasons for concern. None of these trials was designed to look for fracture risk, and arguably none of them was long enough to do so. CREDENCE was stopped early after 2.62 years due to reaching its primary endpoint (a composite of end-stage kidney disease, a doubling of the serum creatinine, or death from renal or cardiovascular causes). If cana increases bone resorption and calcium loss over the course of weeks to months and lowers BMD over a year or two, it might just take longer to lead to result in an increase in actual fractures.
A bit more detail on some of these issues here:
https://www.tandfonline.com/doi/full/10.1080/03007995.2016.1174841
This cohort study finds no difference in fracture risk in patients treated with canagliflozin vs.GLP-1 agonists, but GLP-1 agonists are known to lower lean body mass, so that may not be saying much.
Believe what you will. I am a decades-long user of metformin and it is still near the top of most lists for life extension.
I think between Metformin and acarbose, I don’t need an extra diabetic drug. Especially one that may cause weaker bones. I think my bases are already covered.
I have empaglifozin at home since weeks, but can’t get myself to even try it. ![]() I was hoping to get Canagllifozin first, but seems Empaglifozin has more reliable data. But still… who know taking something you don’t really need in hope that it might help. Taking rapamycin and acarbose seems enough ATM. Why did you decide for Metformin @DeStrider? What won you over? What is your dose?
I was hoping to get Canagllifozin first, but seems Empaglifozin has more reliable data. But still… who know taking something you don’t really need in hope that it might help. Taking rapamycin and acarbose seems enough ATM. Why did you decide for Metformin @DeStrider? What won you over? What is your dose?
I take gliflozins intermittently/as-needed basis, maybe once or twice a week:
(1) towards trough levels of rapa, because I have no idea if there can be any immunosupression while on rapa that would increase the risk of UTI
(2) I don’t take it unless I’m having something sugary, or have a big meal coming up
(3) Yet, I try to take them once a week, because of their cardiac/ renal benefits which could be independent of its glucose lowering abilities.
That makes a lot of sense. What’s ironic about that is that you might otherwise want to take a “rescue” dose of an SGLT2i exactly when you were taking rapa in order to counteract any hyperglycemia during peak PD.
I use acarbose and fiber for rescue, and very occasionally berberine .
I have been taking Metformin for years along with my father and mother. I am prediabetic while my mother is diabetic. I used to take 2 g a day, but ended up having too low blood sugar. Now I take 500 mg daily at night. According to research, the biggest downside of Metformin is slightly decreased exercise performance. My father is a gym rat and he can’t notice any difference pre or post Metformin usage. So, I am going to take the blood sugar and anti-cancer benefits over any negatives that I probably wouldn’t notice. Also, as a Rapamycin user, I need to take either Metformin or Acarbose, and I decided on a bit of both to hedge my bets. Remember that Rapamycin is horrible for diabetes and can shorten a diabetic’s life instead of increasing it if the diabetes is not in control.
Metformin’s decreased exercise performance was what was keeping me away. Mostly this NYT article I guess. My husband was offering me prescription months before as I was trying to loose some BF and he said metformin or glifozins would be great help…
Unfortunately I can’t read it as it is behind a paywall.
Yes, that article makes a strong case for not using Metformin if you exercise regularly. I guess a better combination would be Acarbose + Rapamycin. However, will that be enough to overcome Rapamycin diabetes? I am not 100% convinced.
It seems to be a no-win situation. Do you not take Metformin and suffer from the side effects of Rapamycin diabetes, or do you take Metformin and lose the benefits of exercise? Currently, I don’t exercise much, so the Metformin route is my best bet. However, when I retire and I start attending the gym regularly, I may want to stop Metformin. The bone issue with canagliflozin also seems to be a non-starter.
If you are diabetic, and your blood sugar level is above normal, you should not take Rapamycin.
If you are sedentary, or pre-diabetic, metformin could be used to prevent Rapa-diabetes.
If you exercise regularly you could take Acarbose and Rapamycin to counter Rapa-diabetes.
Those are my thoughts at this moment.
I’ve been through all the posts, so apologies if someone has already mentioned this, but it seems to me that there may be cause for hope regarding Canagliflozin and fractures, and that changes in bone health (as measured by biochemical parameters), in healthy people may be transient (~1wk), but prolonged in diabetics (>26wks) ?
…or maybe I’m just clutching at straws
Blockquote
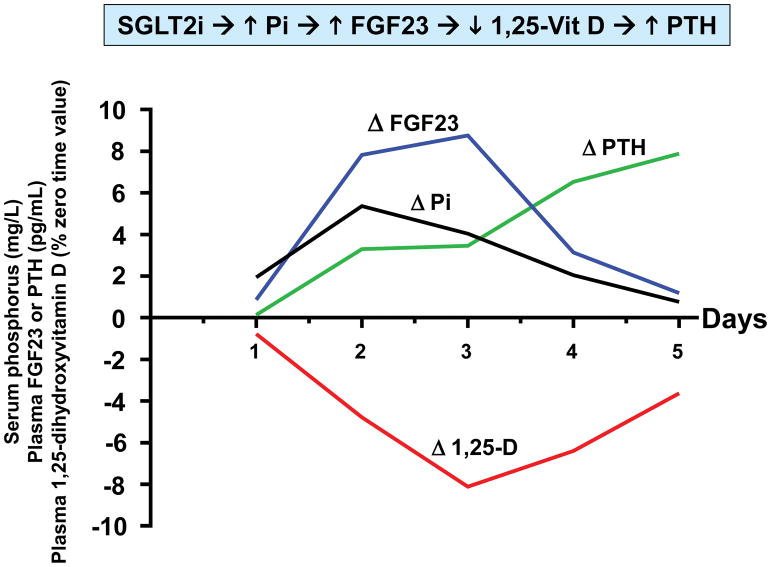
In a recent study of canagliflozin in healthy volunteers, we identified drug-induced endocrine changes that may mediate the drug’s adverse effect on bone health5. Canagliflozin (300 mg/d) increased mean serum phosphorus levels – with a peak increase of 0.61 mg/dL observed after 36 hours of drug administration. The increase in serum phosphorus was correlated with increased transtubular reabsorption of phosphate. This increase in serum phosphorus is reminiscent of electrolyte changes characterizing CKD – albeit changes are smaller in SGLT2 inhibitor-treated patients. Whereas increased serum phosphorus is driven by decreased glomerular filtration in CKD, SGLT2 inhibitors increase proximal tubular reabsorption of phosphate. Nevertheless, both CKD and SGLT2 inhibitors trigger the FGF23/1,25-dihydroxyvitamin D/PTH axis. Plasma FGF23 was increased by ~20% within 24 hours after initiation of canagliflozin (Fig. 1). This was followed by a decrease in plasma levels of 1,25-dihydroxyvitamin D (-10%) and increased plasma PTH (+25%). The decrease in 1,25-dihydroxyvitamin D levels was likely mediated by FGF23-induced decrease in expression of the CYP27B1 gene. We hypothesize that decreased levels of 1,25-dihydroxyvitamin D decrease gastrointestinal absorption of dietary calcium, which in turn promotes PTH secretion. Consistent with this hypothesis, we observed a decrease in 24 hour urinary calcium excretion on days 4 and 5 of our study.
Time course of pharmacodynamic responses to canagliflozin
Healthy volunteers (N=25) were admitted to the NIH Clinical Center to participate in a randomized crossover trial 5. Research subjects participated in two admissions during which they received either placebo or canagliflozin (300 mg/d) for five days. Each research subject was randomized between placebo or canagliflozin for the first admission, and then crossed over to the other treatment for the second admission. Four blood samples were obtained on each day (8 AM, 10 AM, 12 noon, and 8 PM). The details of the study design are described in Blau et al. 5 We calculated averages of the timed blood samples on each of the five days of the study. This figure presents drug-induced changes of those averages (i.e., placebo-subtracted values) for each day. In order to display all four parameters on the same axes, we expressed the data in the following units: serum phosphorus (mg/L); plasma FGF23 (pg/mL); plasma PTH (pg/mL); and 1,25-dihydroxyvitamin D (% of time zero value).
Blockquote
The drug-induced increase in serum phosphorus was transient in our healthy volunteer study. The increase in serum phosphorus was followed by induction of two phosphaturic hormones (FGF23 and PTH), which function in tandem to restore serum phosphorus levels toward normal. Levels of FGF23 and 1,25-dihydroxyvitamin D were also restored toward baseline, but PTH levels remained elevated at the end of our five day study. In contrast to our data with healthy volunteers, the mean increase in serum phosphorus (≈ 0.15 mg/dL) was sustained for at least 26 weeks in type 2 diabetic patients treated with canagliflozin. The mean increase was greater (≈ 0.35 mg/dL) in diabetic patients with impaired renal function (mean eGFR = 39 mL/min/m2). Just as the increase in serum phosphorus was sustained in diabetic patients, we hypothesize that canagliflozin-induced changes in FGF23, 1,25-dihydroxyvitamin D, and PTH are also sustained in diabetic patients.
Blockquote
The block quotes and graph are from the paper