More is not necessarily better, but nobody can answer your question definitively because there aren’t any health outcome studies with SGLT2i drugs in healthy subjects. 25mg is slightly better than 10mg at increasing glucose excretion in the urine, but most in the life extension community are taking it for its potential pleiotropic benefits.
FWIW, empagliflozin at 12.5mg/day did absolutely nothing for my glucose levels in the 5 months I’ve taken it. I bumped it up to 25mg/day, and re-tested several times in the 6 months at that dose, and while it did nothing for my A1c (5.7-5.8), it reliably brought my FBS to under 100mg/dL (95-98), while previously it had been at 105-112 mg/dL (dawn effect).
Based on that I’m sticking to the 25mg/day dose. I see no downsides to that dose and rather like the possible greater glucose excretion at the higher dose. There are no advantages to the lower dose that I know of - initially I was at a lower dose because I didn’t know how my body would react to empagliflozin, so I wanted to start at a lower dose in case of an unexpected negative reaction (i.e. a safety precaution).
But of course you should test and monitor the effects in yourself, as all of us are unique.
The biggest upside to 12.5 mg vs 25 mg is that your supply lasts twice as long. I split the 25s. Based on what I’ve seen, the results are similar between the doses and you get most of the bang for the buck in the initial dose compared to doubling it.
Just published, low dose might be as good if not better: Real-world outcomes and safety of low- vs. standard-dose SGLT2 inhibitors in heart failure 2025
After matching, 46,218 patients remained (23,209 in each group) and baseline characteristics were well-balanced (absolute standardized differences < 0.1). The primary outcome incidence was 6.0 per 100 person-years in both groups. After multivariable adjustment, Low-dose SGLT2i was associated with a lower risk of the composite outcome (HR 0.93, 95% CI 0.88–0.99, p=0.02) and CV hospitalization (HR 0.84, 95% CI 0.78–0.91, p<0.0001), with no significant differences in HF hospitalization (HR 0.93, p=0.33) or mortality (HR 1.04, p=0.47). The incidence of genital mycotic infections and other adverse events was similar in both groups.
Ah another new article that says the opposite lol: Off-label low-dose therapy in heart failure reduced ejection fraction: can we achieve the benefits of the four pillars?
However, SGLT2 inhibitors showed a trend to improve outcome in target dose. However, follow-up echocardiography (n=415) showed that an improvement in LVEF of ≥10% was more frequently observed in patients who had achieved the target dose of each medication. (Target dose RASi= 79.4%, BB= 72.5%, MRA=61.8%, and SGLT2i=64.8%
Other good articles:
In a real-world study of older adults with heart failure, empagliflozin and dapagliflozin use was associated with a lower risk of incident cognitive impairment.
Our study provides strong evidence supporting causal, protective effect of SGLT2 inhibition against stroke, particularly ischemic stroke, based on MR. The consistent findings across diverse GWAS exposures and robust sensitivity analyses underscore therapeutic potential of SGLT2 inhibition in stroke prevention. However, high heterogeneity in the overall model and broader stroke subgroup warrants further studies.
The pooled analysis showed that dapagliflozin significantly reduced office SBP (MD -3.08 mmHg, 95% CI -3.45 to -2.71; p < 0.00001; I² = 0%; Figure 1A) and office DBP (MD -1.10 mmHg, 95% CI -1.86 to -0.34; p = 0.004; I² = 0%; Figure 1B). Similar reductions were observed in 24-hour SBP (MD -3.53 mmHg, 95% CI -5.03 to -2.03; p < 0.00001; I² = 0%; Figure 2A) and 24-hour DBP (MD -2.13 mmHg, 95% CI -3.58 to -0.67; p = 0.004; I² = 11%; Figure 2B).
Dapagliflozin suppresses vascular smooth muscle cell senescence by promoting autophagy 2025
This study reveals that Dapa could suppresses vascular aging via promoting autophagy.
Empagliflozin and dapagliflozin showed no significant difference in the primary outcome (13.8% vs. 14.8% in LVEF≤ 40%, 5.5% vs. 5.3% in 40<LVEF<50%, 4.8% vs. 6.0% in 50%≤LVEF), without heterogeneity (P for interaction=0.777).
This study suggests that dapagliflozin and empagliflozin have similar clinical outcomes in the management of HF across all ranges of LVEF in a real-world setting.
46 drug-naïve adults with T2D (HbA1c 6.5 %–10.0 %) received empagliflozin (10 mg/day; n = 23) or metformin (1000 mg/day; n = 23) for 12 weeks
Empagliflozin significantly reduced mean amplitude of glucose excursions (MAGE) compared to metformin in drug-naïve individuals with type 2 diabetes.
Time-in-range (TIR) improved significantly in both groups, but only empagliflozin demonstrated superior reduction in glucose variability.
Empagliflozin led to greater improvements in body weight, waist circumference, triglycerides, and HDL-cholesterol levels than metformin.
Has this been discussed here? For all-cause mortality (ACM), canagliflozin is ranked first and dapagliflozin second. I don’t have access to read the full study.

SGLT2 inhibitors were associated with a disproportionately higher reporting frequency of bladder cancer events compared to metformin, dipeptidyl peptidase 4 inhibitors, or glucagon-like peptide-1 receptor agonists. MR analysis results showed that SGLT2 inhibition was associated with a higher risk of bladder cancer (odds ratio 1.01; 95%CI 1.00-1.01; p = 0.004).
Bad news? But the OR seems very tiny (1.01): is that clinically significant? What do you think @Neo?
It kind of makes sense in that you are dumping sugars (cancer and bacteria food) directly into your bladder.
Herein, we identified previously unknown fasting-induced, glucagon-mediated inhibitory effect of the circadian clock gene basic helix-loop-helix ARNT like 1 (Bmal1) on the expression of the main proximal tubule glucose transporter solute carrier family 5 member 2 (Sglt2) in mice. During fasting, glucagon induces Bmal1, which increases expression of nuclear receptor subfamily 1, group D, member 1 (Rev-erbα). Rev-erbα represses nuclear respiratory factor 1, a transcriptional activator of Sglt2, and diminishes Sglt2 expression and thereby kidney glucose reabsorption capacity. During refeeding (lower glucagon) this process is attenuated, thereby inducing glucose reabsorption.
Summary : During fasting, glucagon release inhibits glucose reabsorption by inhibiting SGLT2. So it seems that dual GLP1 and Glucagon agonists (survodutide, mazdutide, retatrutide ) are also milder SGLT2 inhibitors. It will be interesting if studies ever compare those dual glucagon agonists to SGLT2 inhibitors.
I did not realize this, but glucagon becomes elevated during fasting. Glucagon releases glucose, and does not allow glucose reabsorption in the muscles because we need that glucose for energy while fasting.
Coincidentally, I was just investigating this confluence of biomechanisms, because I had to take a bunch of blood tests in preparation for my upcoming surgery. There were a few abnormalites flagged as values ouside of the range of the UCLA lab. One was serum chloride at 107 mmol/L (range 96 - 106) with blood sodium within range 144 mmol/L (range 135 - 146), and serum potassium out of range at 5.4 mmol/L (range 3.6 - 5.3). Meanwhile on the urine test, there was glucose present in high amounts +4 (obviously - I take empagliflozin) with normal blood glucose (95 mg/dL), and trace ketones, both flagged as “abnormal”.
In any case, upon investigation, there are a number of possible causes of such values, including dehydration, fasting, intense exercise which depletes glucose stores in muscles, use of SGLT2i which dumps glucose in fasting conditions leading to elevations in glucagon and ketones. There’s a whole bunch of papers I could cite, but the bottom line is this: if you exercise intensely (I do), fast (in my case daily TRF with overnight fasting 20-22 hours), or are on a ketogenic diet, you need to be careful with SGLT2i use before surgery, particularly if you are a T2DM concurrently using glucose lowering agents, particularly insulin (I’m not, and I don’t). The danger is euglacemic diabetic ketoacidosis (eDKA) which can result in death. It is recommended that you stop the SGLT2i at least 3 days before surgery, but longer is better (SGLT2i associated metabolites can persist in the body for 10+ days). Based upon my test results and various papers, out of an abundance of caution, I intend to stop my empagliflozin 12 days before the surgery (somewhat drastic, but given the potentially drastic consequences - death - I’d rather be extra cautious).
In any case, there are very interesting interplays between all these factors, especially impacting kidney health, so anyone taking a SGLT2i should be aware of them, and educate themselves in this subject matter.
Yes seems very tiny. Do you know what the MR’s odd-ratio is per?
In patients with albuminuric CKD, dapagliflozin lowered AASI independently of BP control and sodium handling, suggesting favorable vascular remodeling in both diabetic and non-diabetic patients.
DAPA and EMPA showed parallel effects on glycolipid metabolism, liver and kidney function.
“the reporting frequency of SCD [sudden cardiac death] in the SGLT2 inhibitors group was significantly lower than that of the control group (1.43 vs 4.70/1000; p < 0.001), with a PRR of 0.30 (95%CI 0.26-0.35)”
“These drugs suppress pro-inflammatory M1 macrophage activation, promote anti-inflammatory M2 phenotypes, and reduce the release of cytokines (e.g., IL-1β, IL-6,TNF-α) through pathways such as NF-κB, AMPK/mTOR, and JAK/STAT. In cardiovascular diseases, they attenuate atherosclerosis (AS) and myocardial fibrosis by limiting macrophage infiltration and foam cell formation”
Dutch, Chinese, Saudi paper: Antidiabetic Medications and Bladder Cancer Risk in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials 2025
- This meta-analysis of RCTs includes the largest evidence base to date (n = 169,534 participants) on this topic.
- DPP-4i, GLP-1Ras, and SGLT-2i are not significantly associated with an increased risk of bladder cancer in T2DM patients.
- The long-term safety of these antidiabetic drug classes are supported by current high-quality randomized trial data.
Interesting only one study in the meta analysis looking at bladder cancer risk in people using pioglitazone. However it was highly elevated risk of 14 events / 2605 vs control group of 6 events / 2633 and OR of 2.37. But to me it is suspect since it is a relatively small study and the bladder cancer incidence in controls is 3 x higher than other studies just glancing over the list. To me this suggest there may be confounding factors in the population they studied.
Also you have an occasional outlier SGLT2i study showing increased risk but when combined with the other studies the differences average out. OR ranges are huge for the majority of studies meaning the sample sizes need to be much larger to really get a meaningful answer since in most studies the range crosses the 1.0 mark.
Hence the meta analysis that in this case is quite useful and convincing. Makes me wonder about the pioglitazone data. I’d love to see a huge meta on pio like this but it seems people have generally stopped using it since the prevailing view is that it slightly increases the risk of bladder CA.
I discovered that I needed to drink a lot more water with Empa. Not quite as bad with Dapa.
It’s hard to change lifetime hydration habits.
There are quite a few studies that show bad things correlated with sodium over 141 mmol/L.
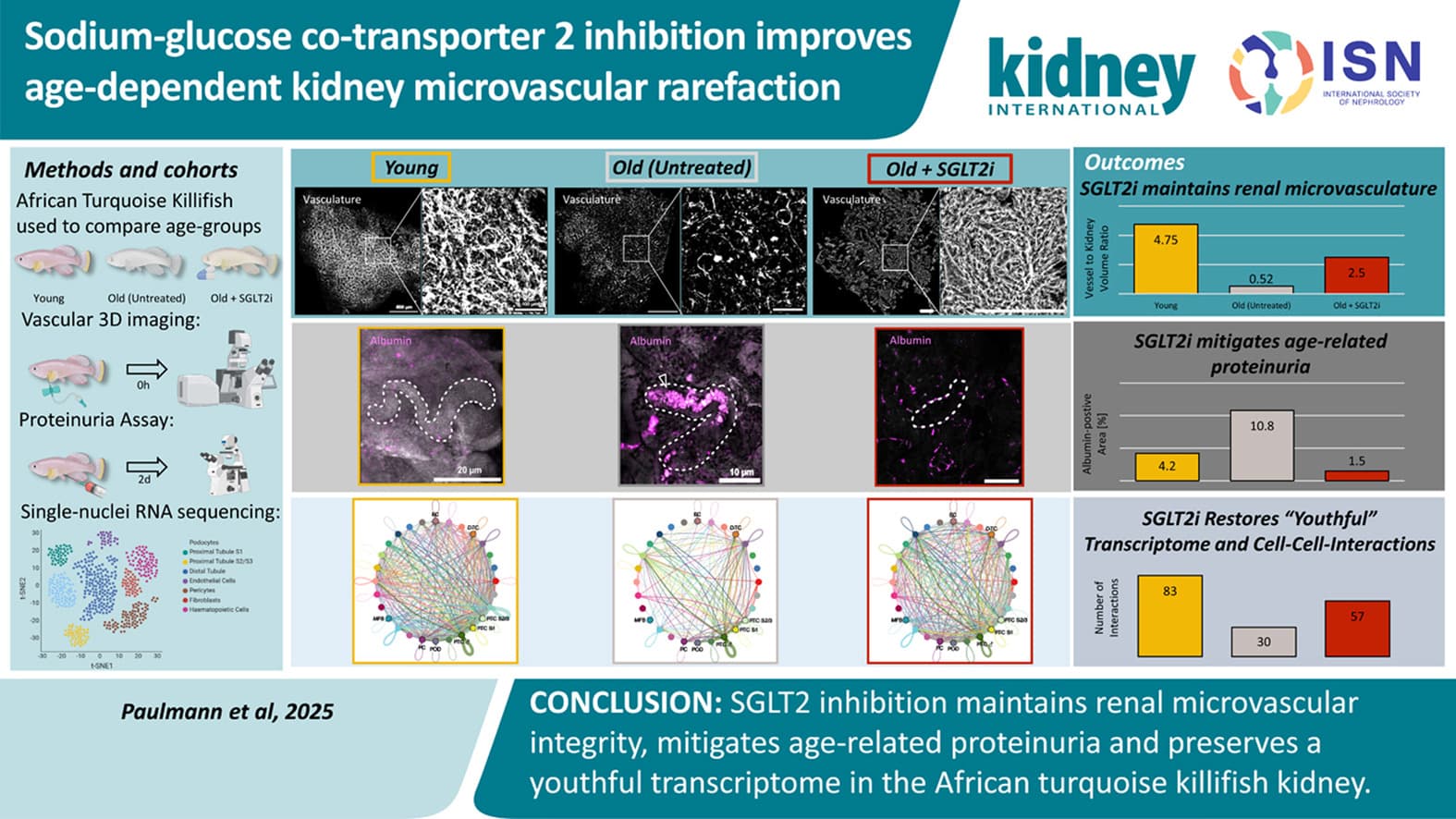
Sodium-glucose co-transporter 2 inhibition improves age-dependent kidney microvascular rarefaction 2025
Our study establishes the killifish as a translational model for investigating kidney vascular aging. SGLT2i preserves kidney microvascular structure and function, reduces proteinuria, and maintains a more youthful transcriptome. These results support a vascular-protective role of SGLT2i in mitigating age-related kidney deterioration.
Is there an indication as to the definition of low dose? It is probably the lowest in the recommended range. I’m taking half of the minimum recommended dose of Canagliflozin and would like to see if there is any research on it. My BG was already within the normal range. I’m taking it primarily to slow the standard rate that even healthy kidney function declines. In that regard Canagliflozin and Telmisartan form functional reno-protective bookends.